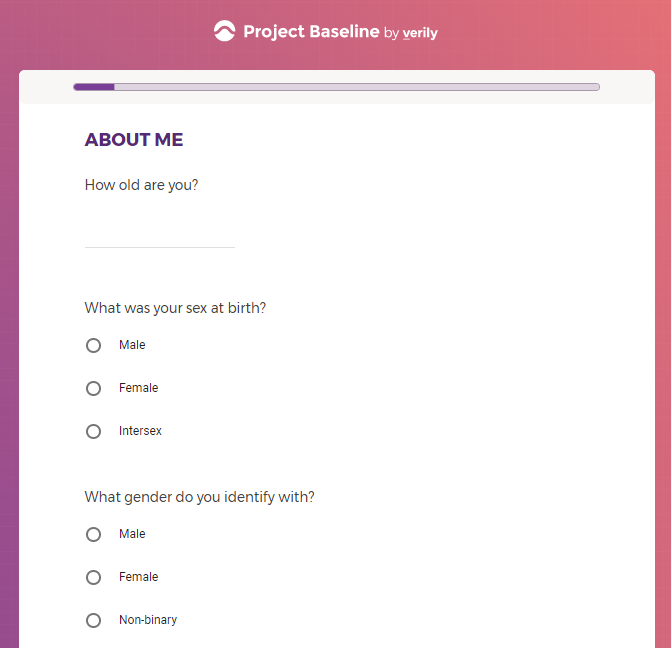
This research began after the White House announced that Google
was building a website for COVID-19.
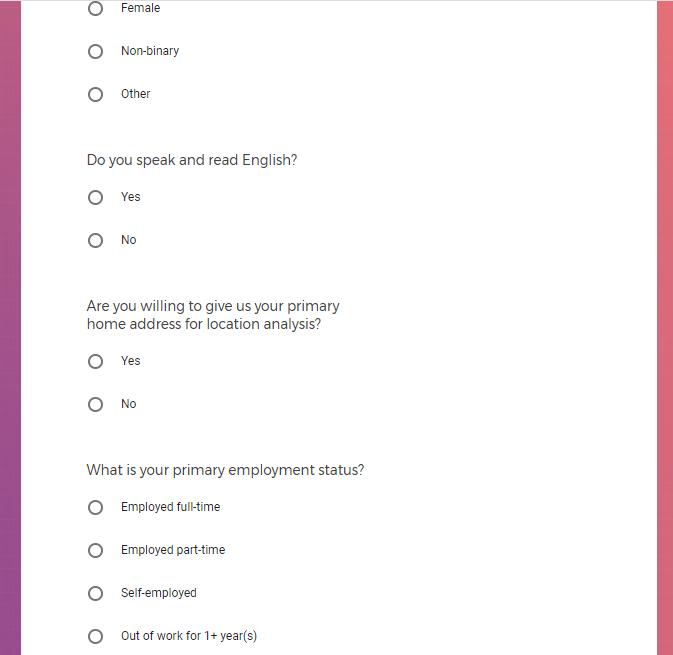
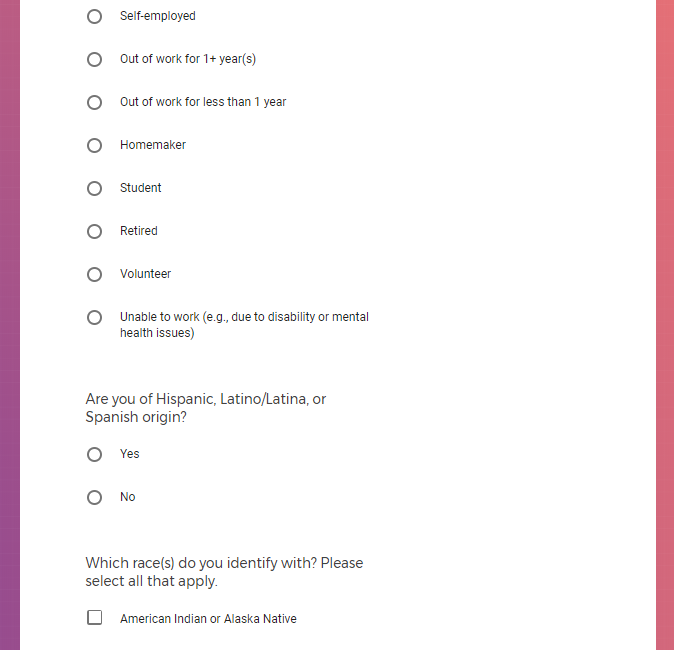
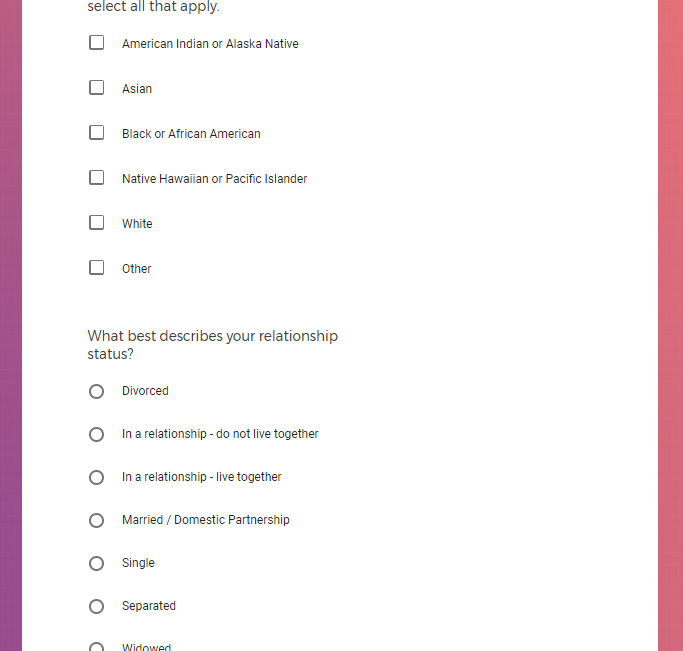
It contains screenshots of platform used to collect highly sensitive personal and medical data.
(Images overlap due to the long web pages -- sorry)
This is posted on an unpublicized section of my website for preview by potential members of an Advisory Board
in the event that this experiment is continued or it's initial findings are referred to an appropriate entity.
Content will expire on April 5th, 2020.
Announced by the White House
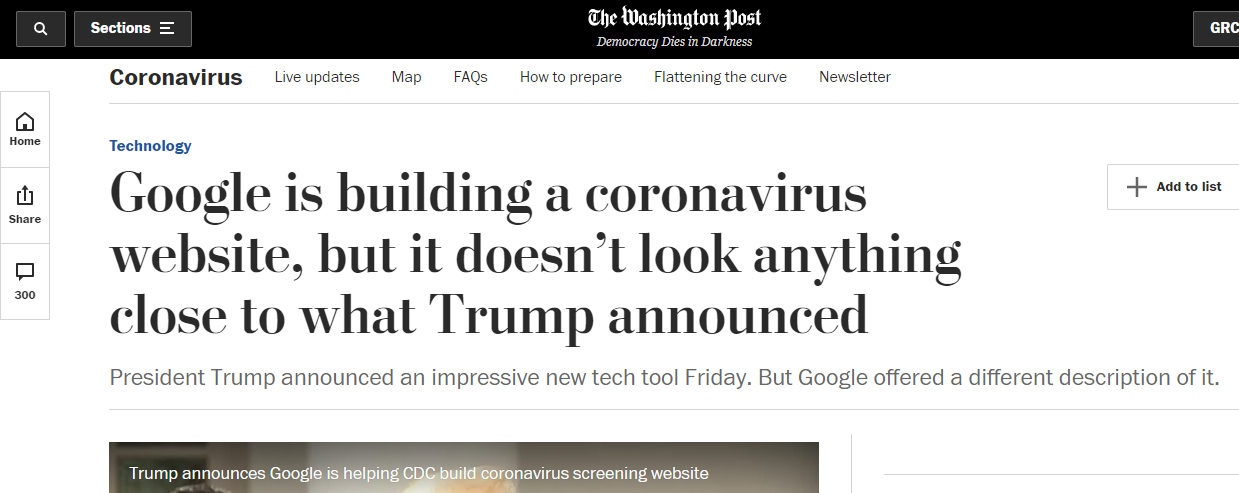
Verily is owned by Alphabet

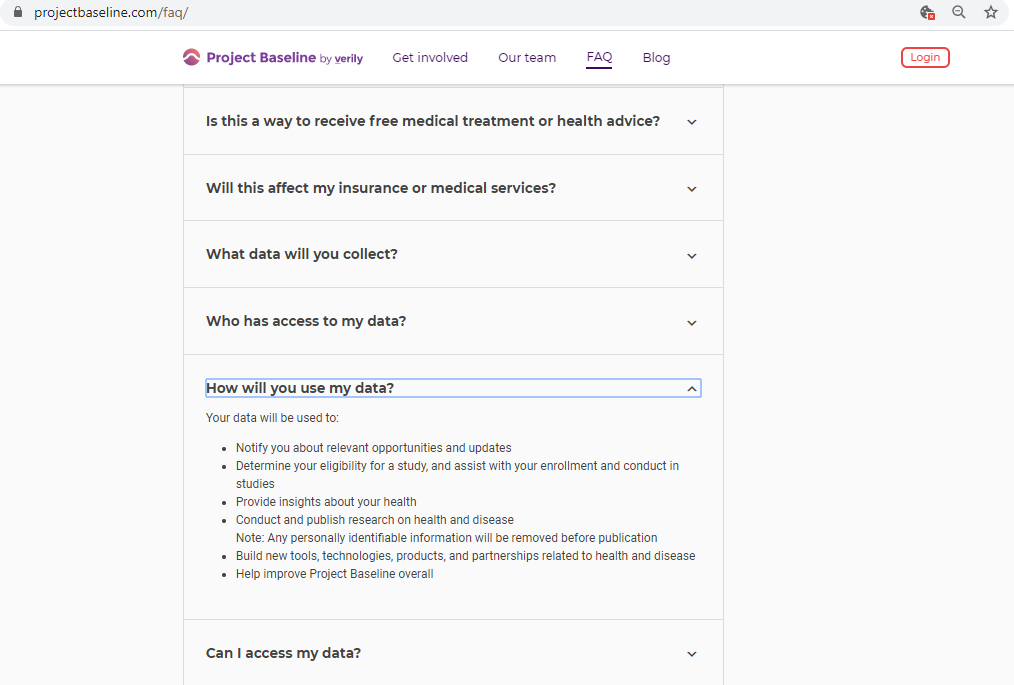
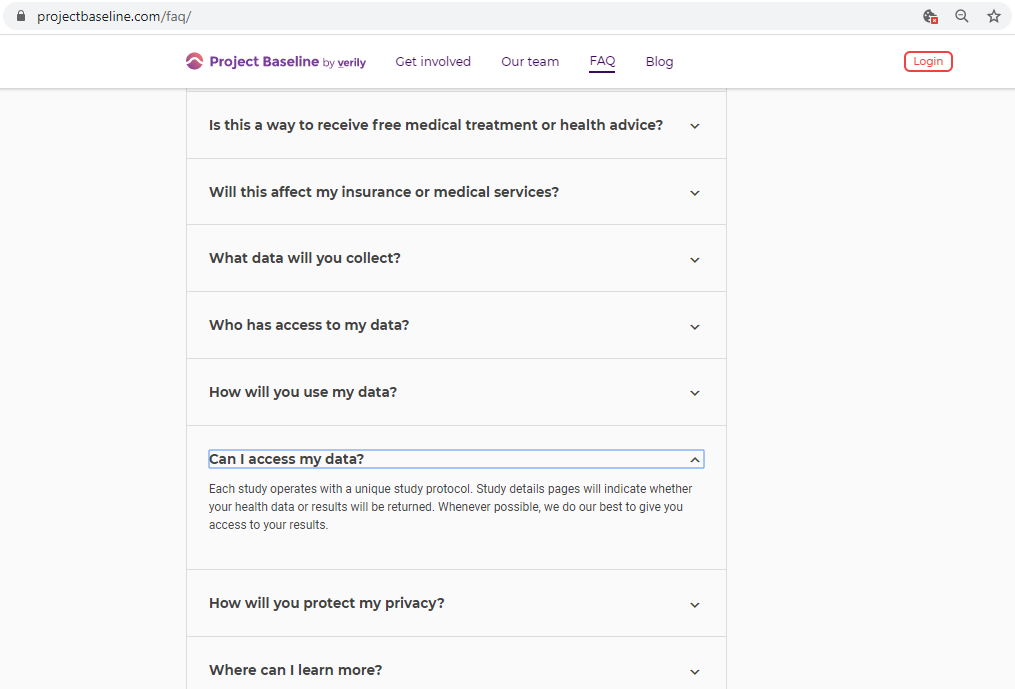
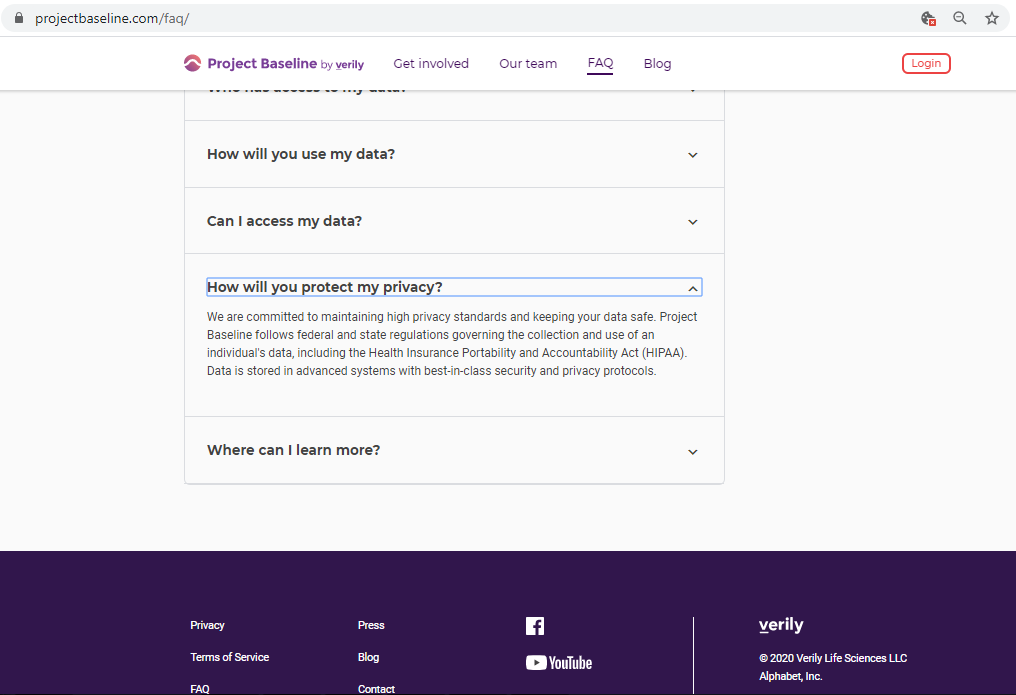
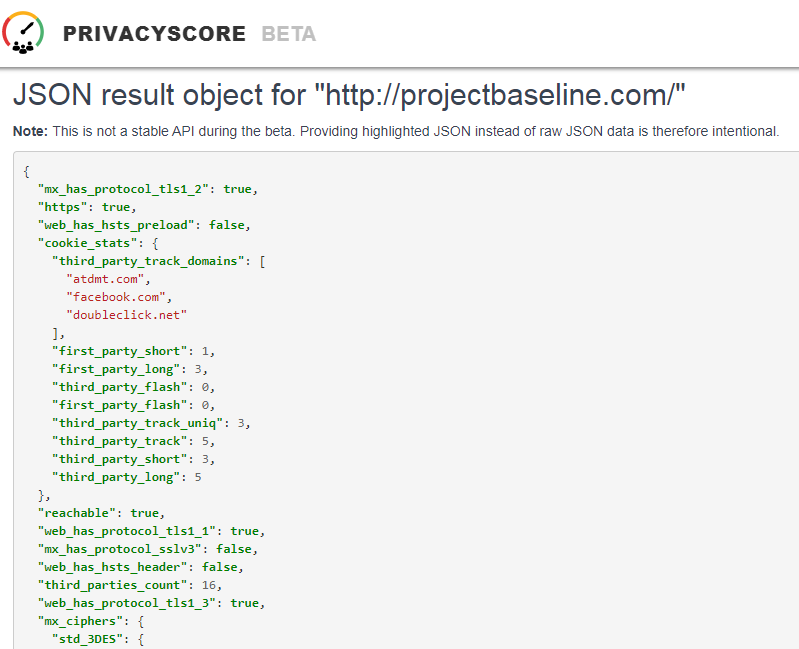
Before submitting personal data to any organization, I always conduct a Vendor Risk Assessment.
Every vendor assessment includes, at minimum, capturing their policies and conducting a privacy scan of cookies, third-parties, and basic security hygiene.
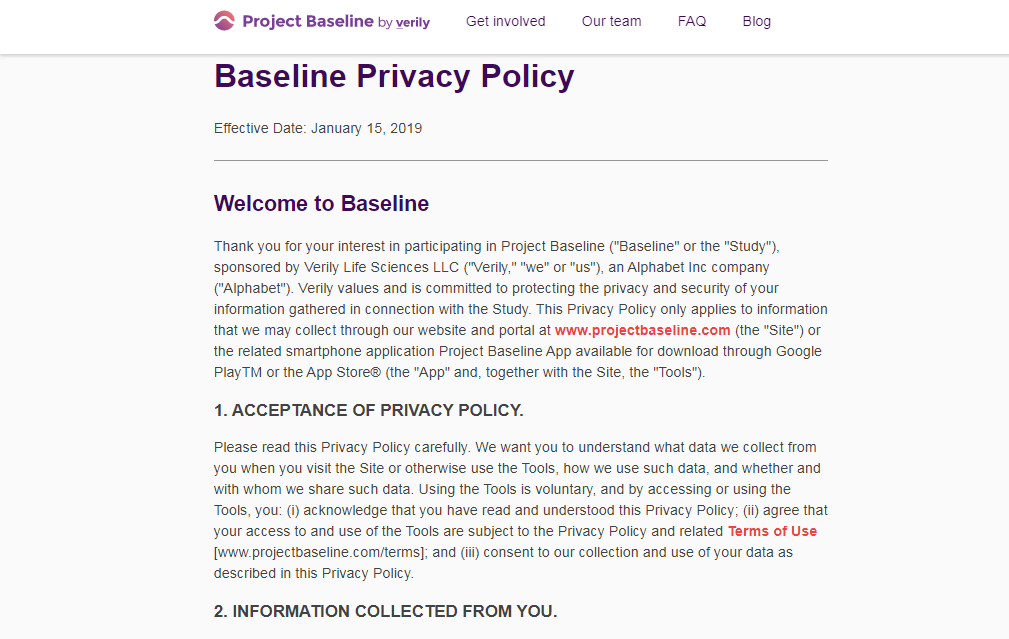
A privacy policy's "fine print" is often found in a separate Terms of Service document.
There are no clauses in Verily's Terms of Service preventing me from collecting information on Project Baseline,
and sharing this information with my own healthcare providers and the general public.
This tool provides a simple baseline.
Additional tests help determine if the organization complies with its own policies, and applicable law.
Experts Voice Concerns

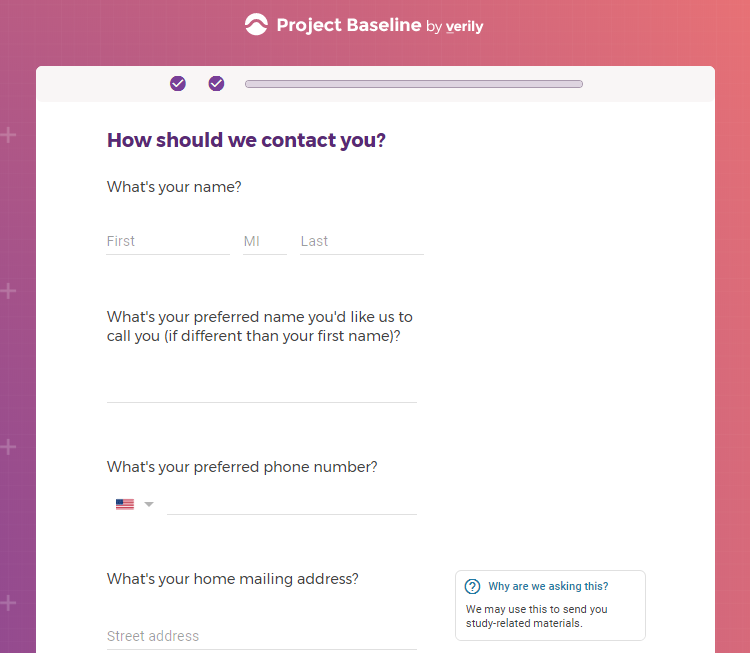
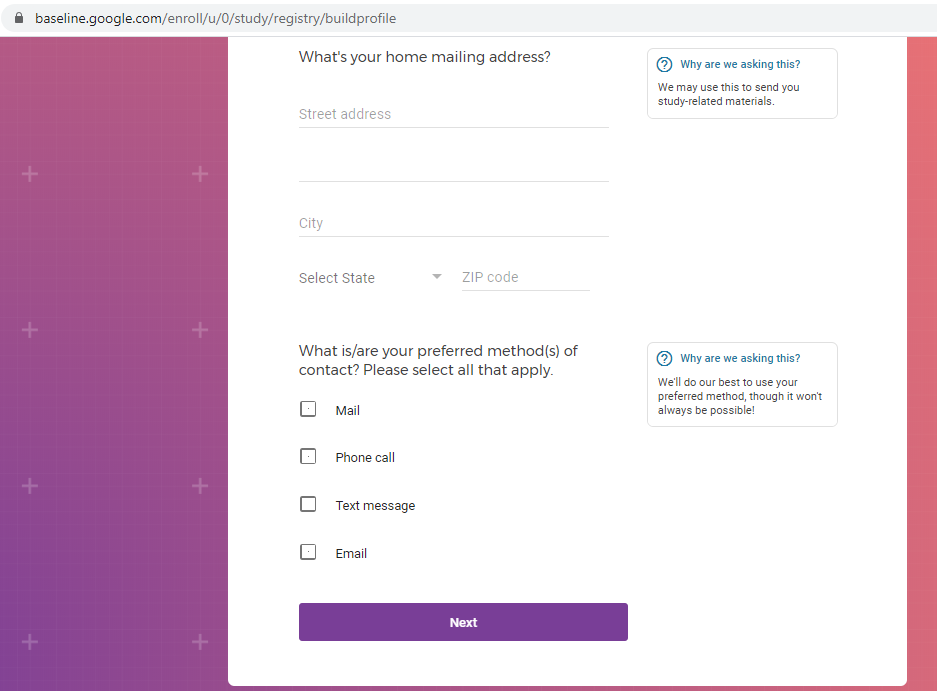
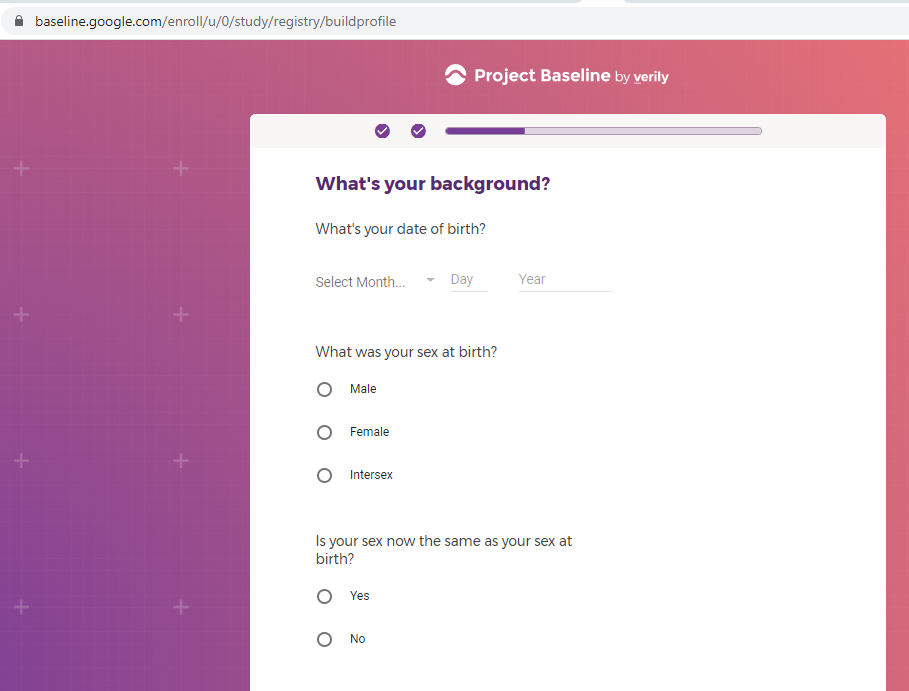
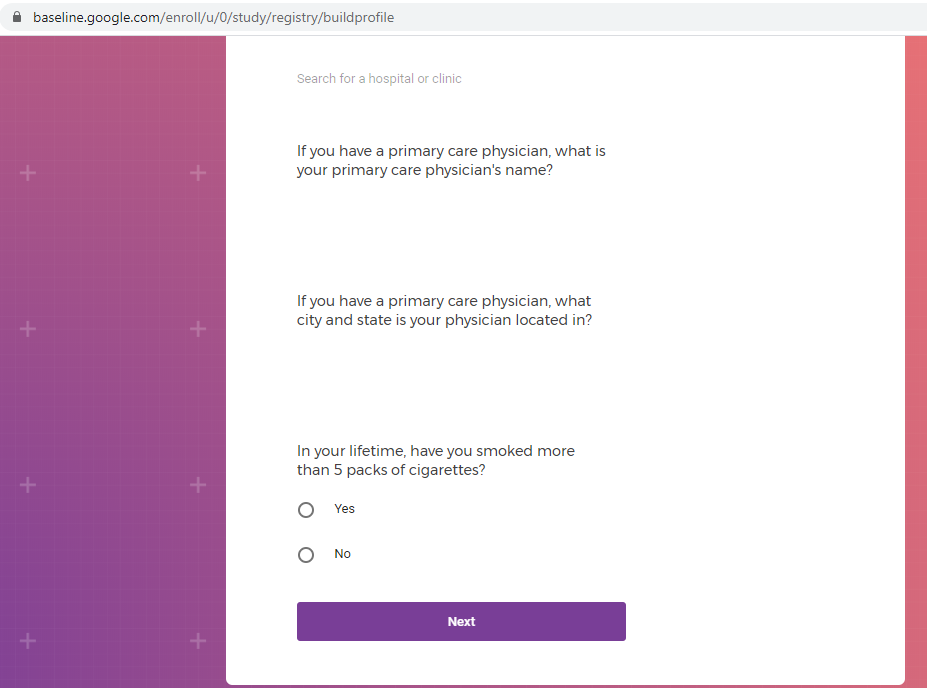
I registered as a participant a day in advance of the COVID-19 site release, thinking I'd get first access to COVID-19 testing.
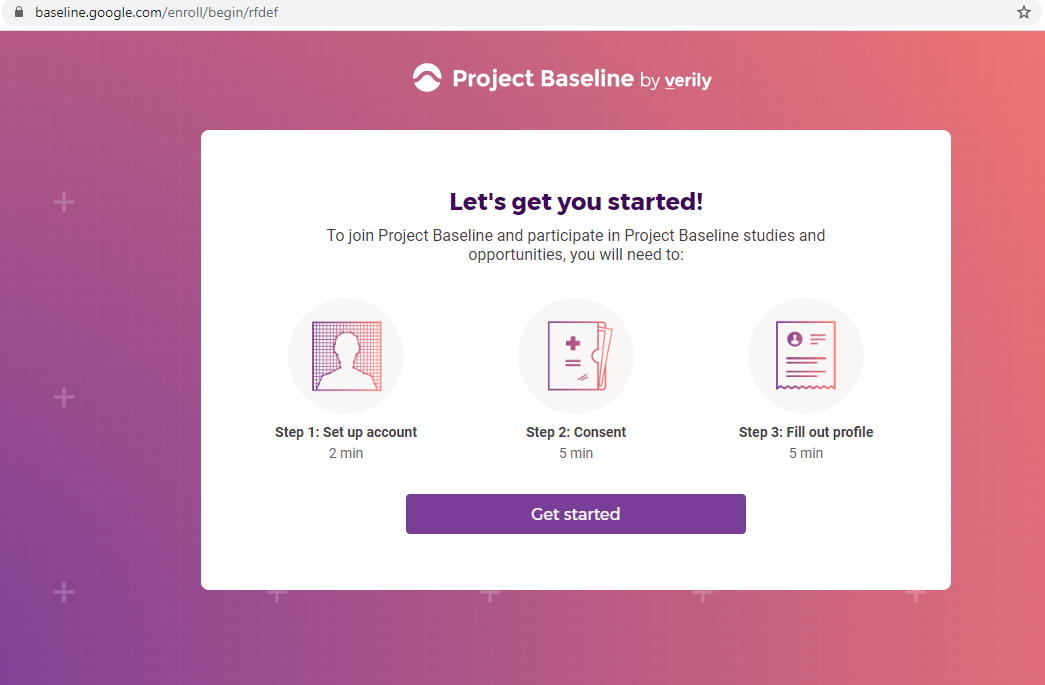
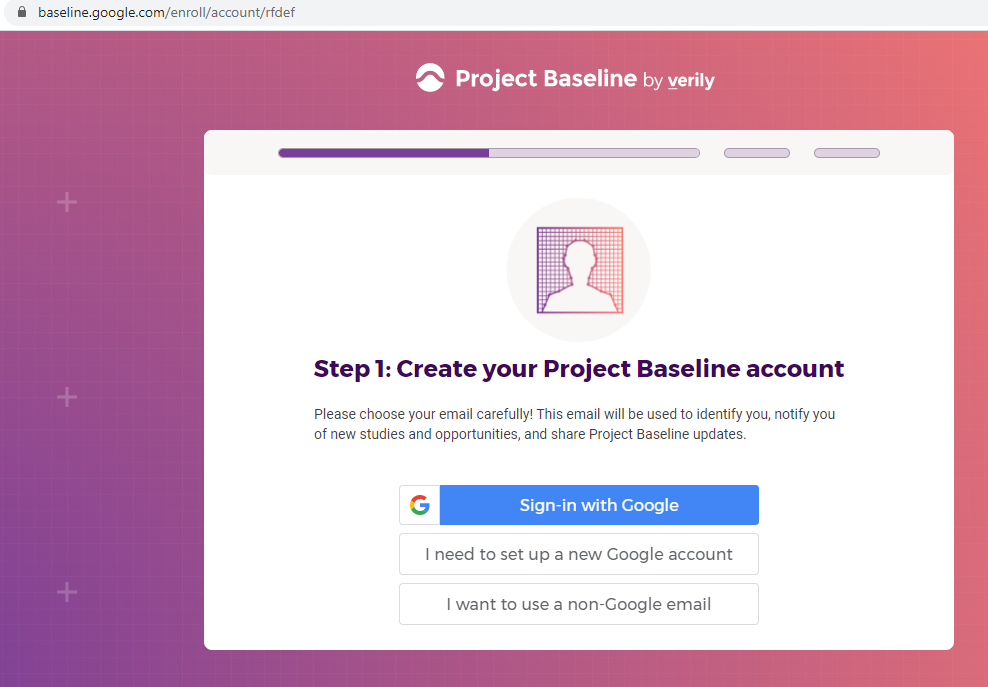
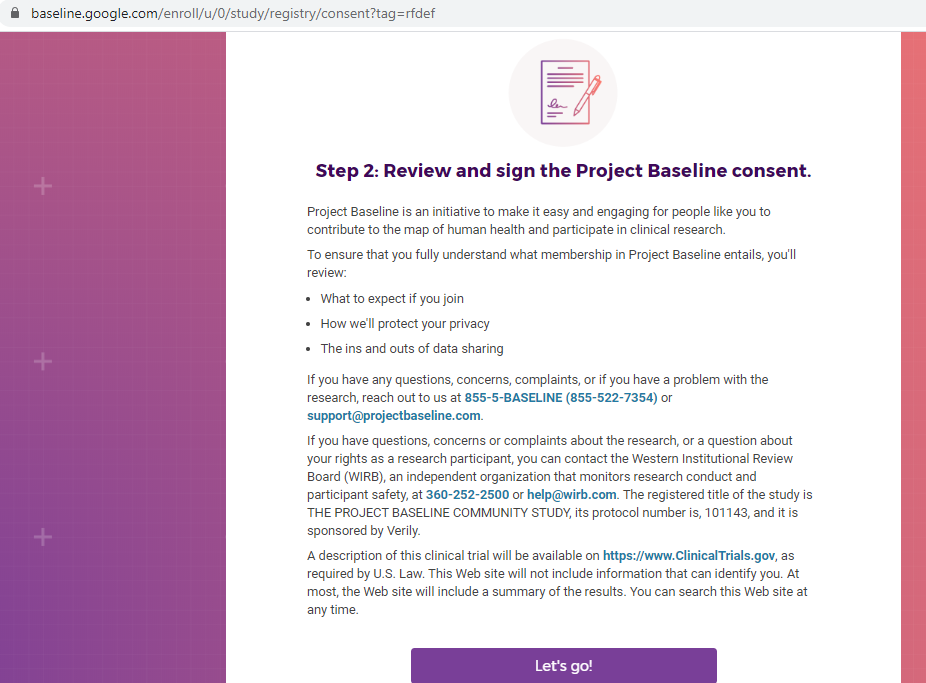
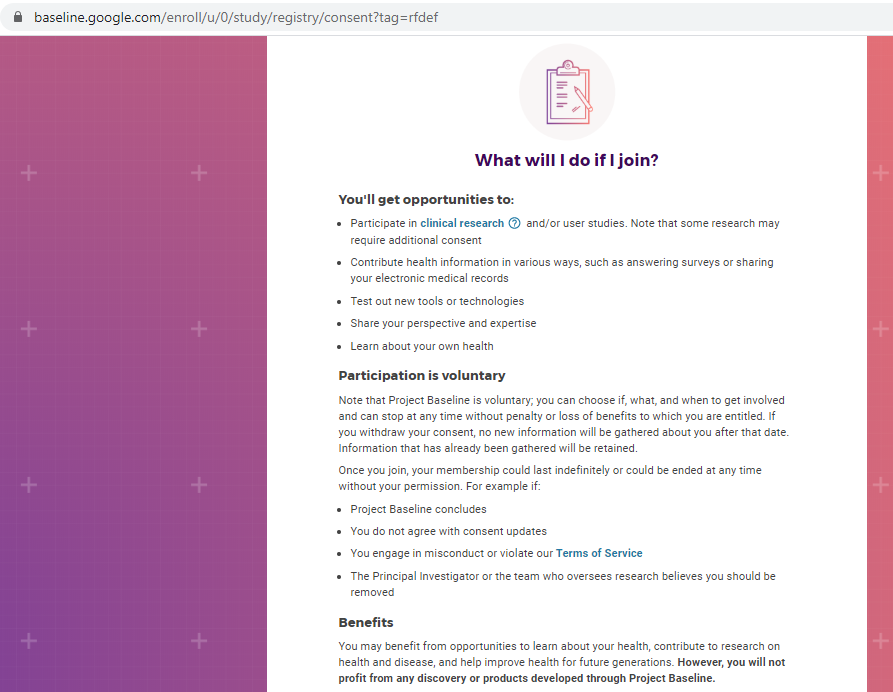
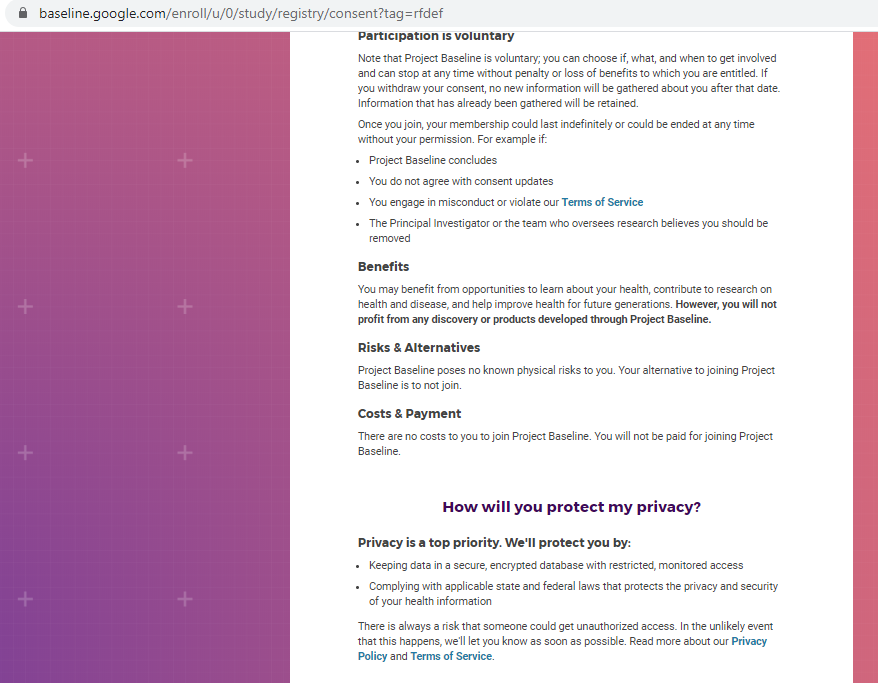
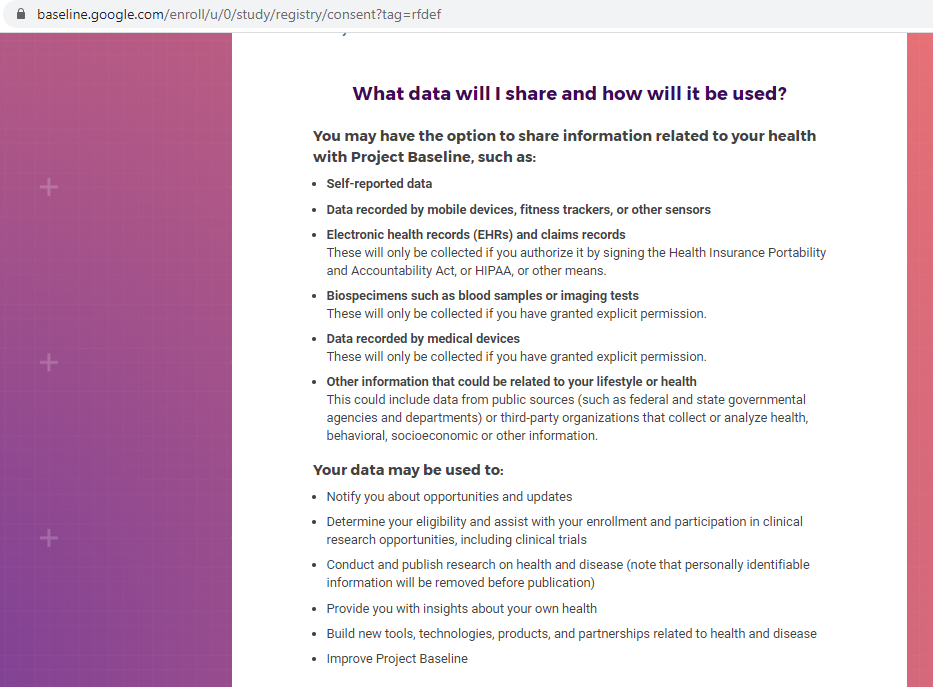
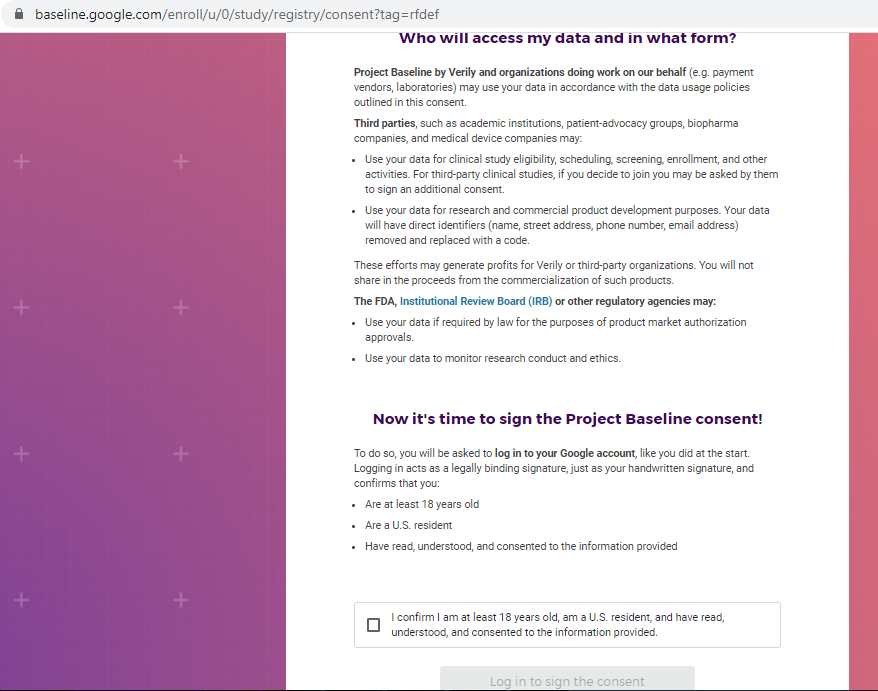
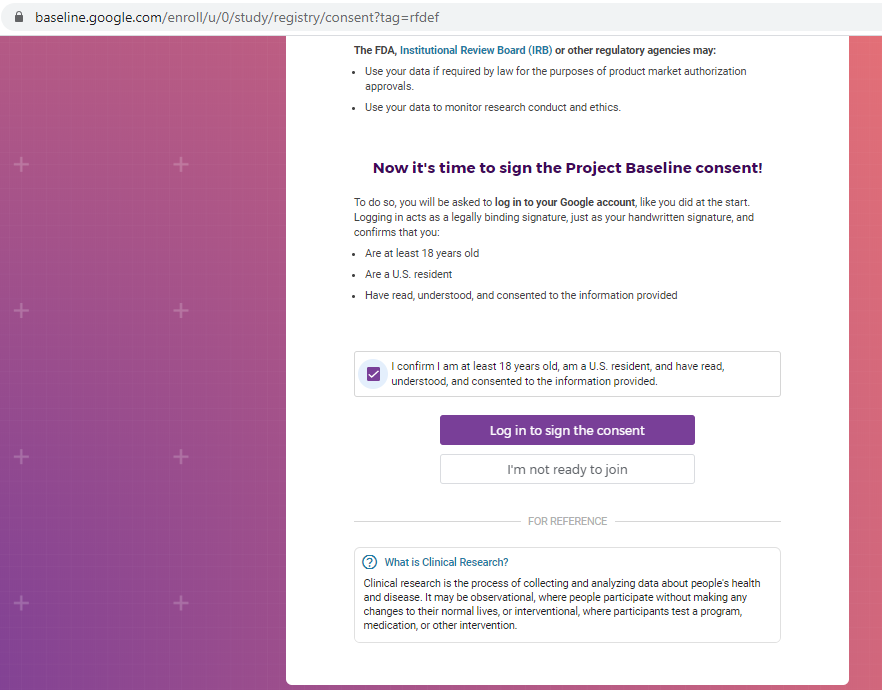
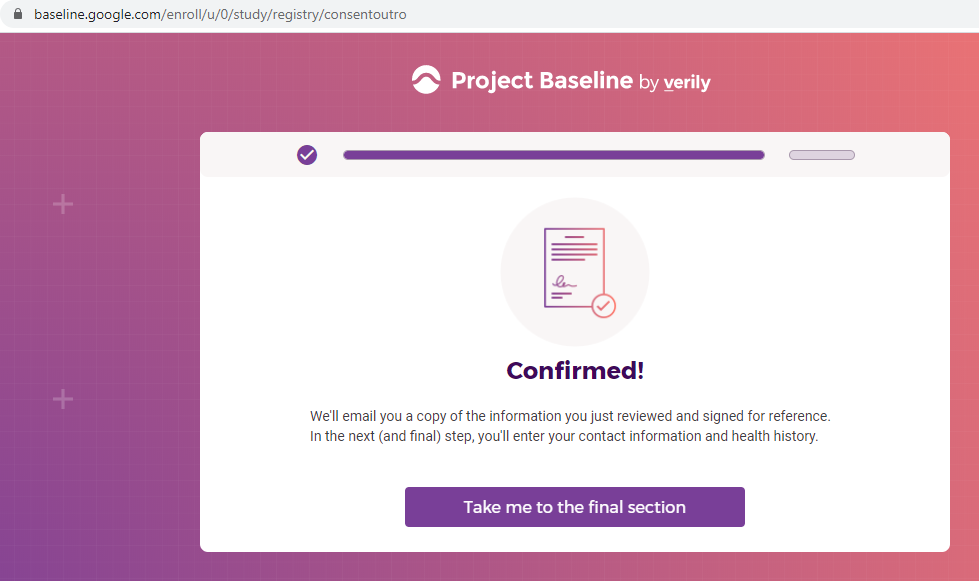
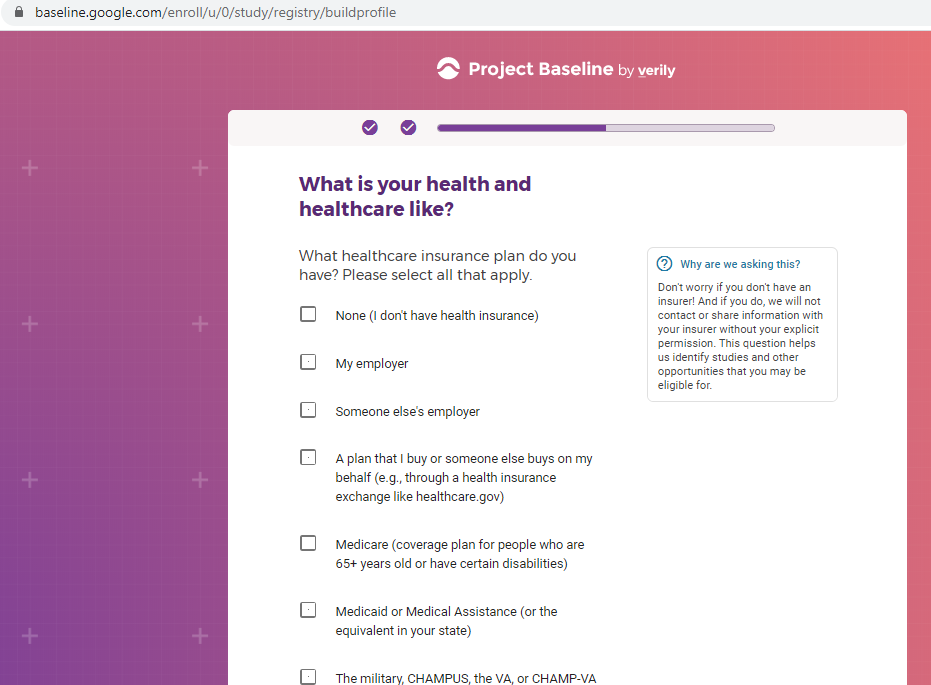
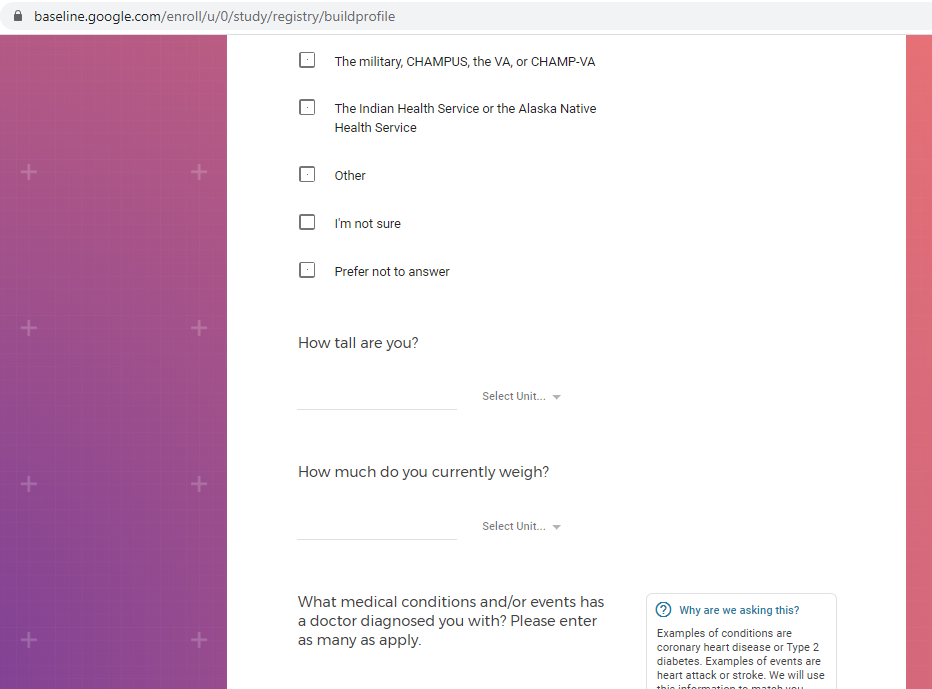
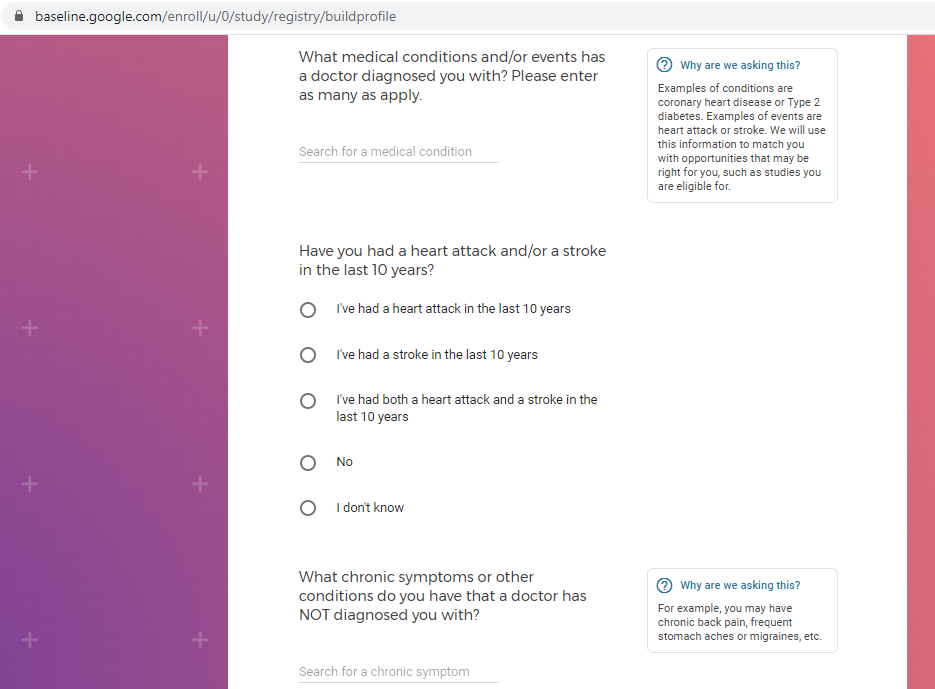
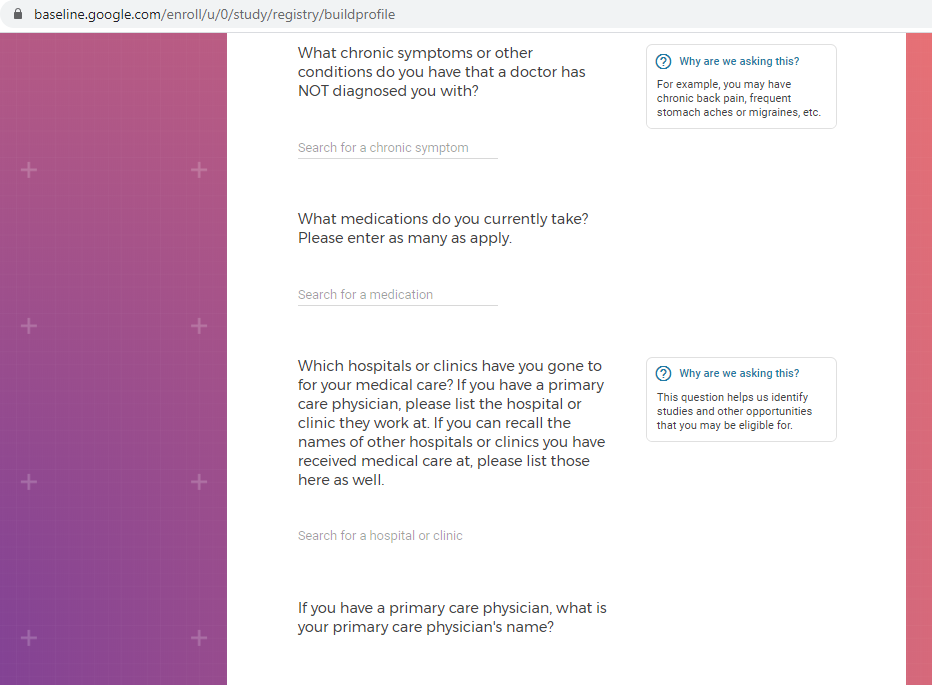
Previous screenshots show the registration process is hosted on the baseline.google.com domain.
While an option does exist for a non-Gmail contact method, I didn't test that.
The link between my gmail account and Verily's Project Baseline has been established.
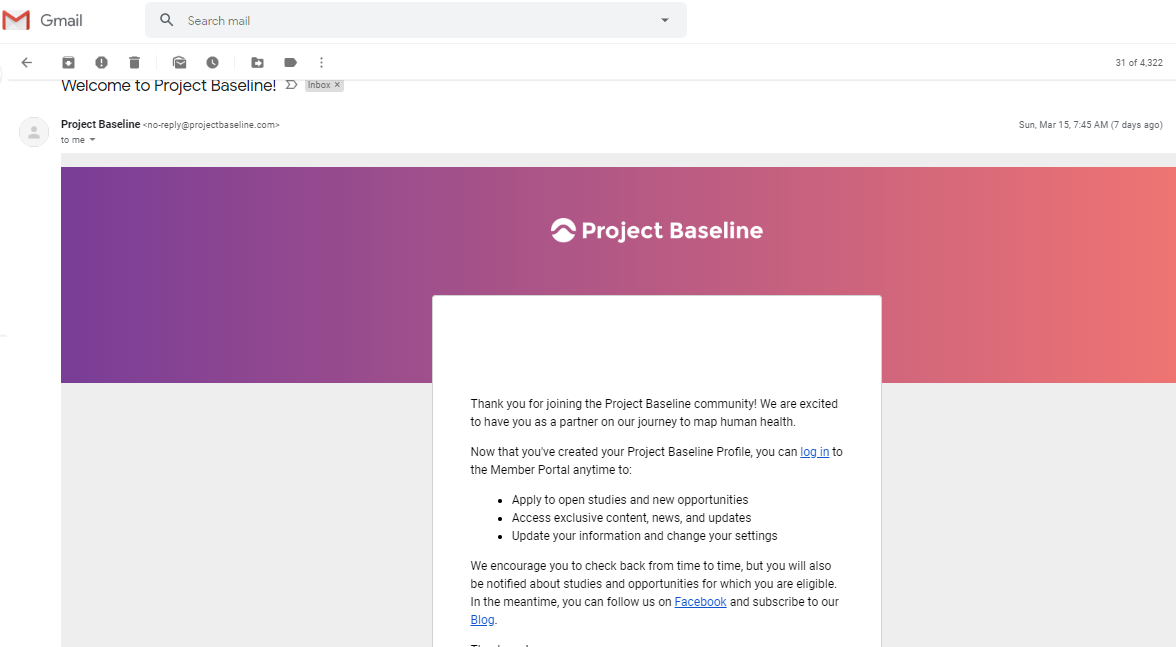
Another email provides a link to the consent I provided:
Normally I like getting a Consent Receipt.
This one is shared storage on https://drive.google.com/file/d/1ipmF1lYNSs5c-L98_1l-OR8Ypt2syYaH/preview,
which I will compare against the consent I provided according to the evidence gathered.
At 4:20am PDT, I tweeted the link where the White House's notorious flowchart ends:
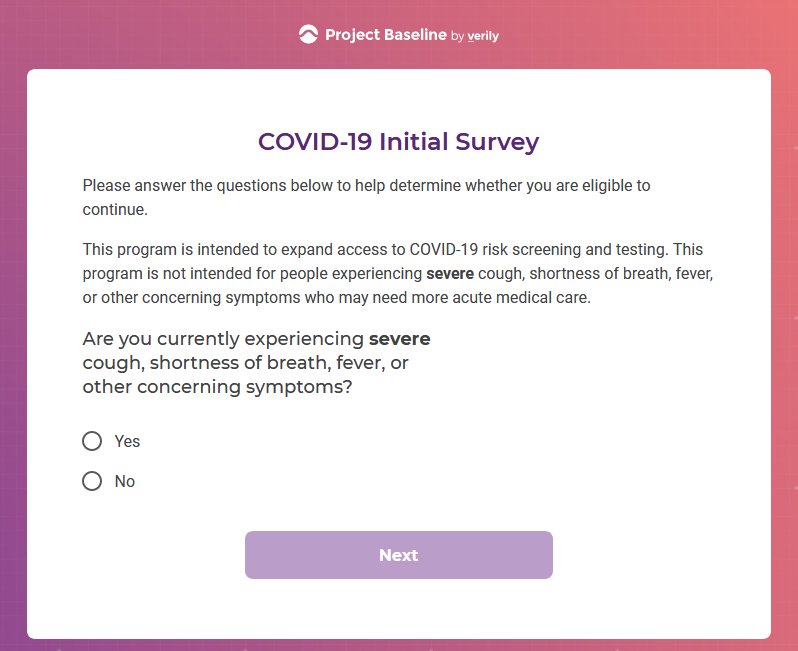
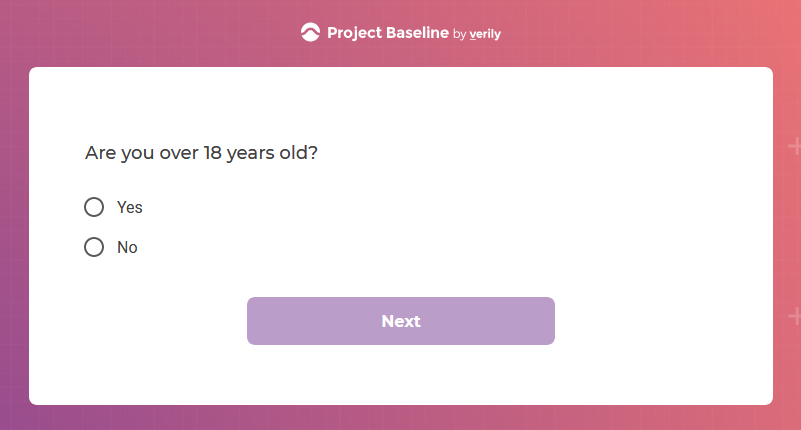
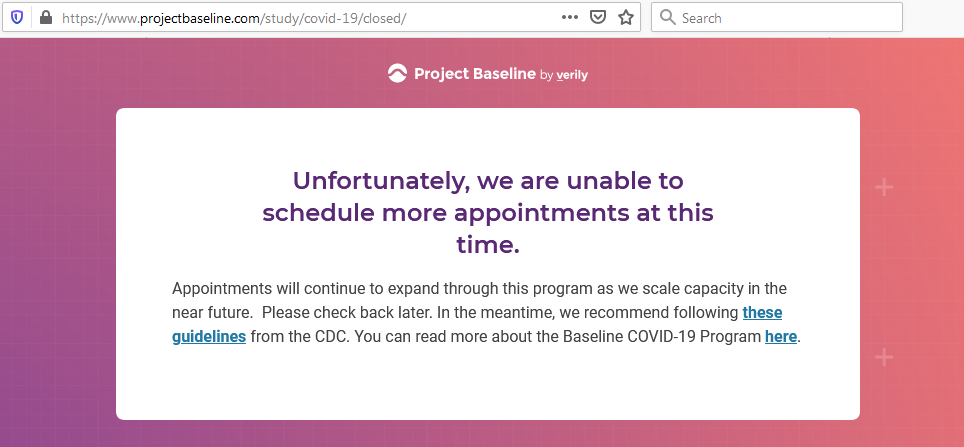

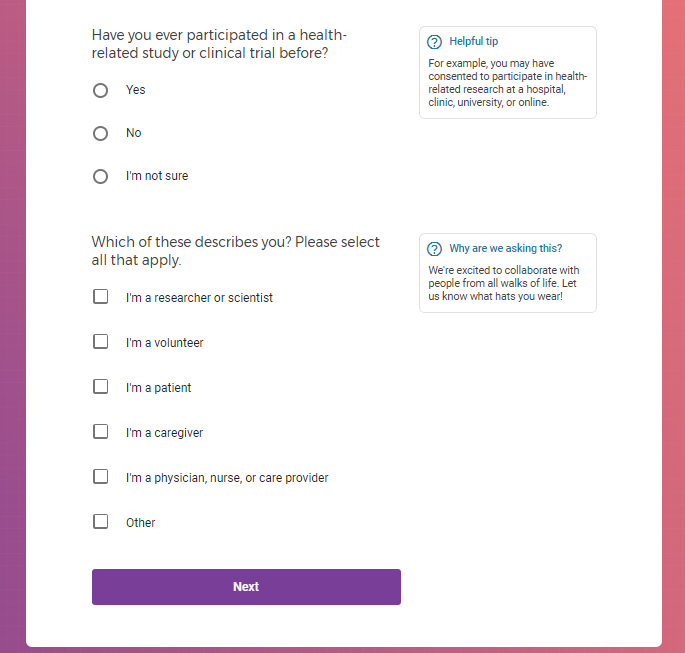
I tested the hypothesis that being a participant would get me access to such studies and services...
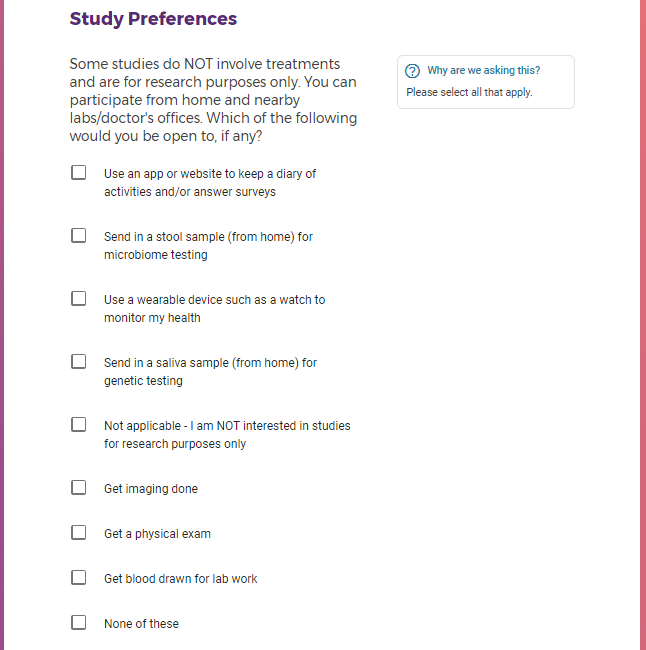
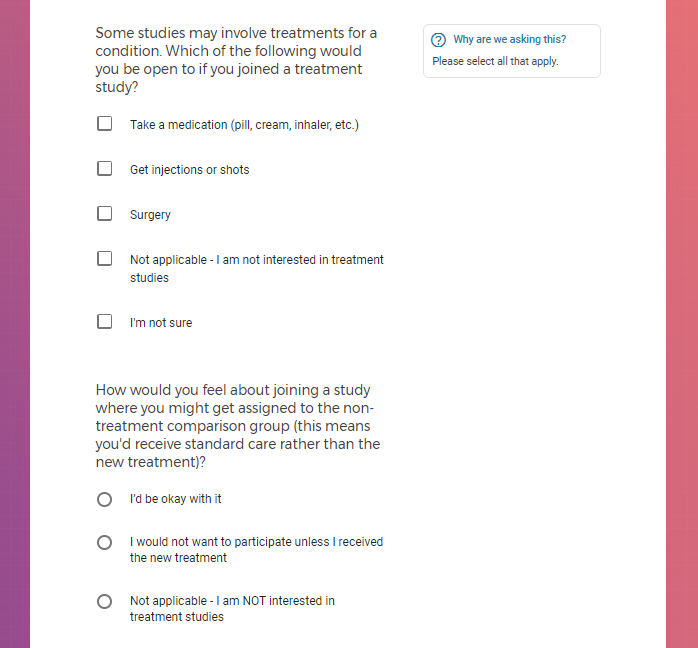
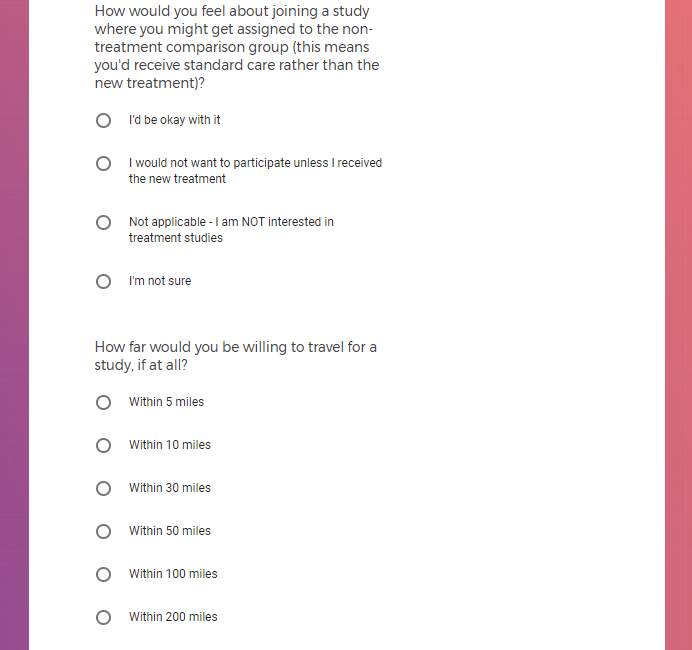
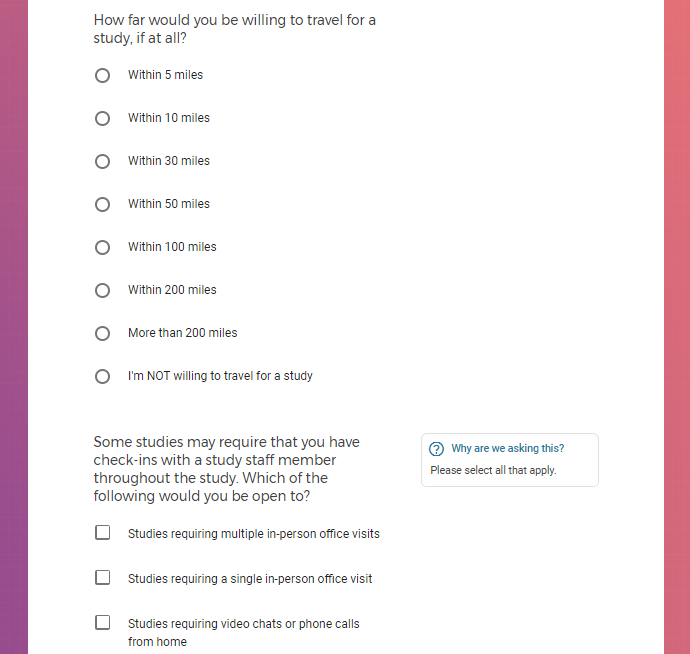
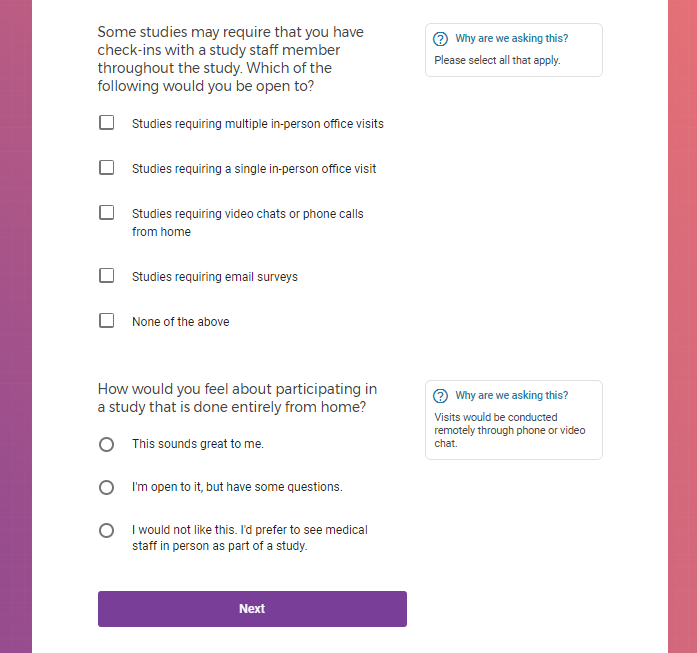
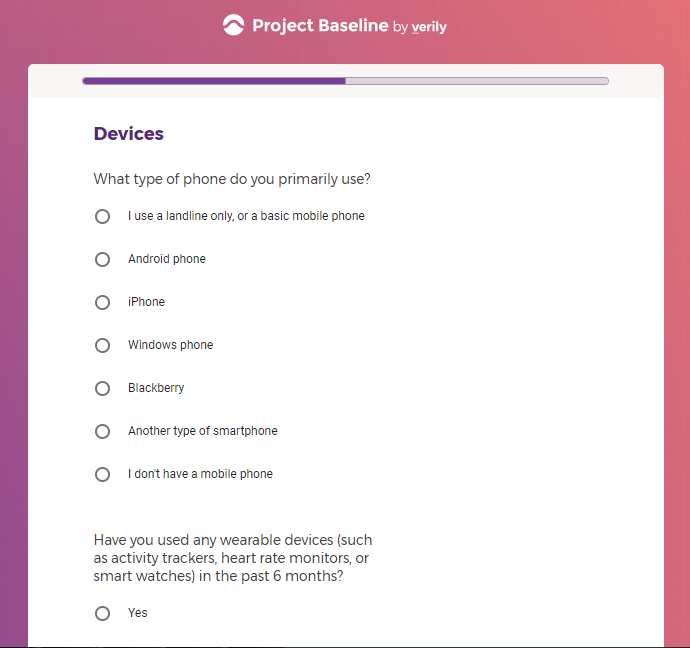
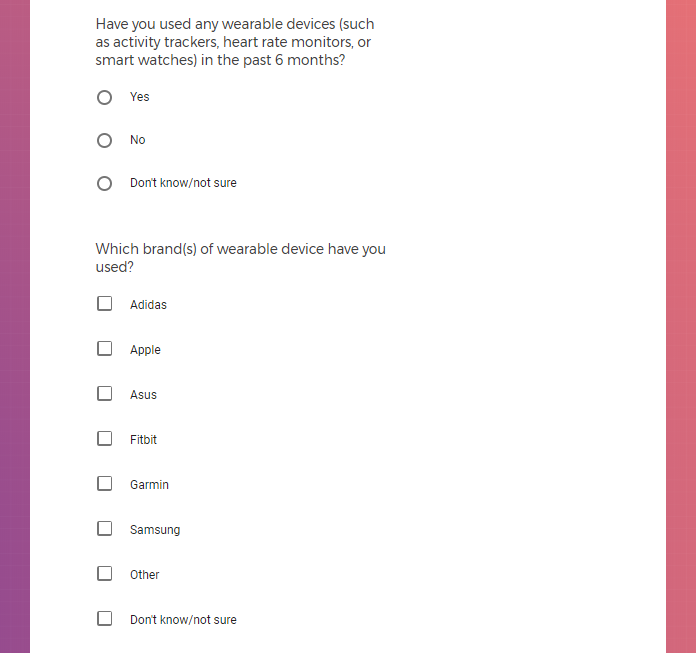
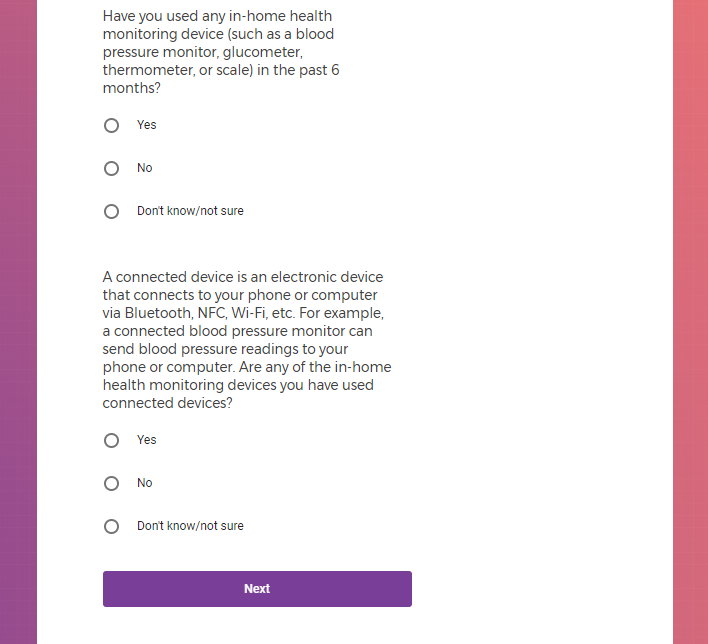
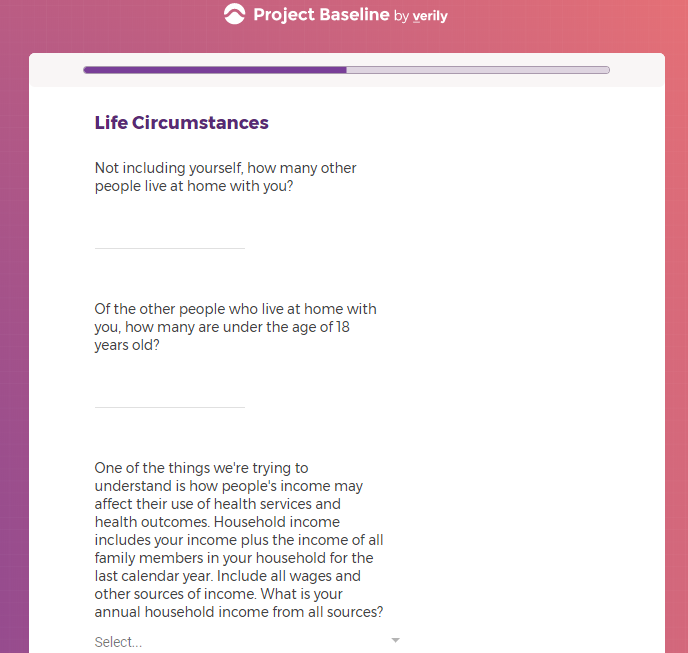
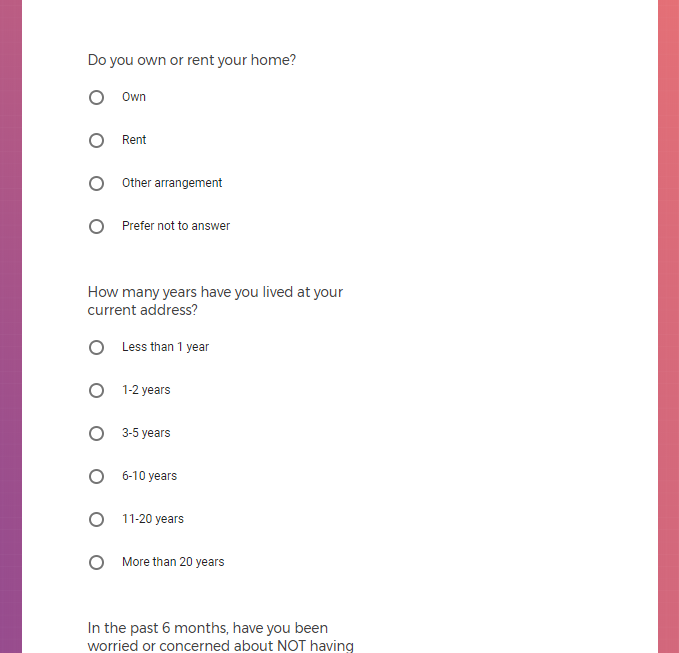
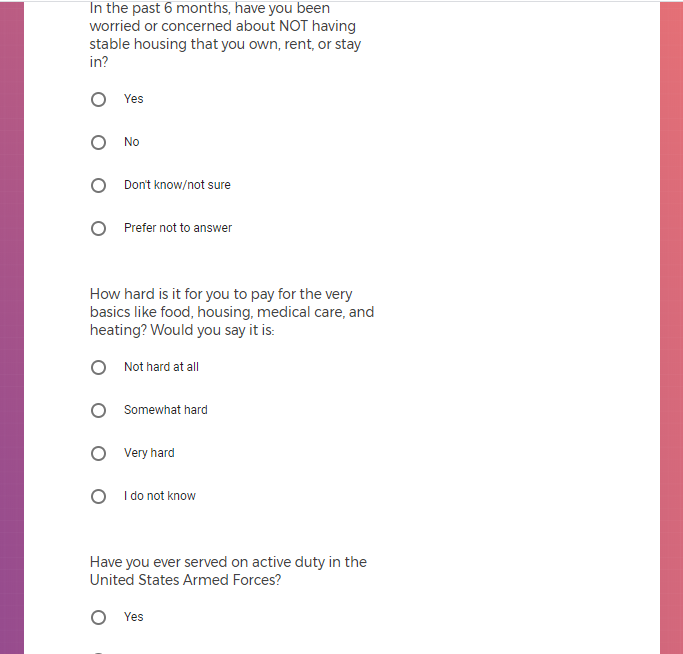
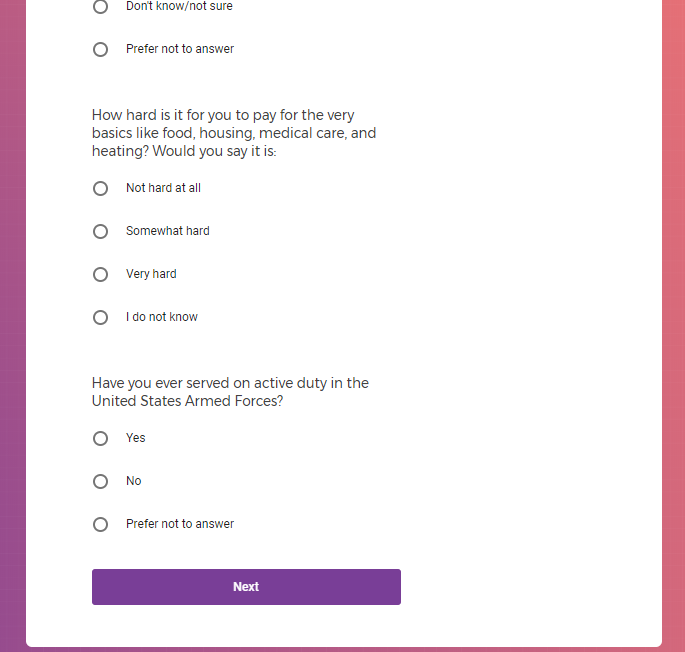
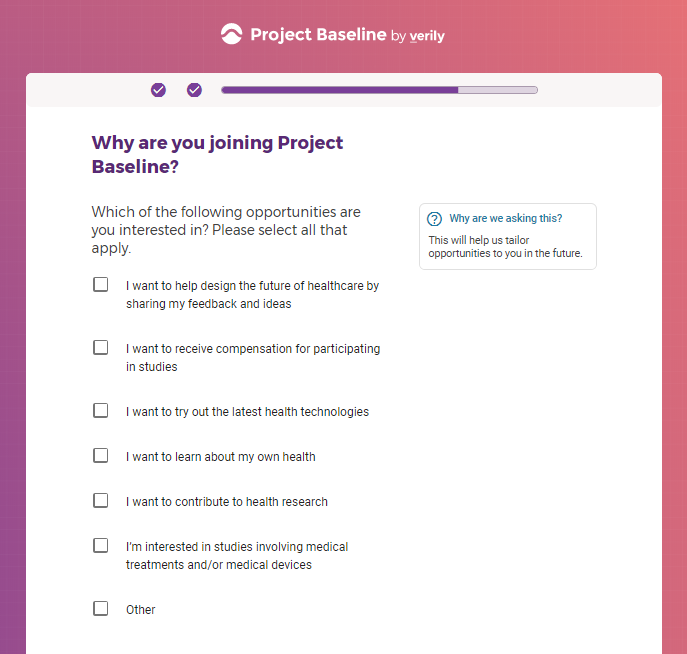
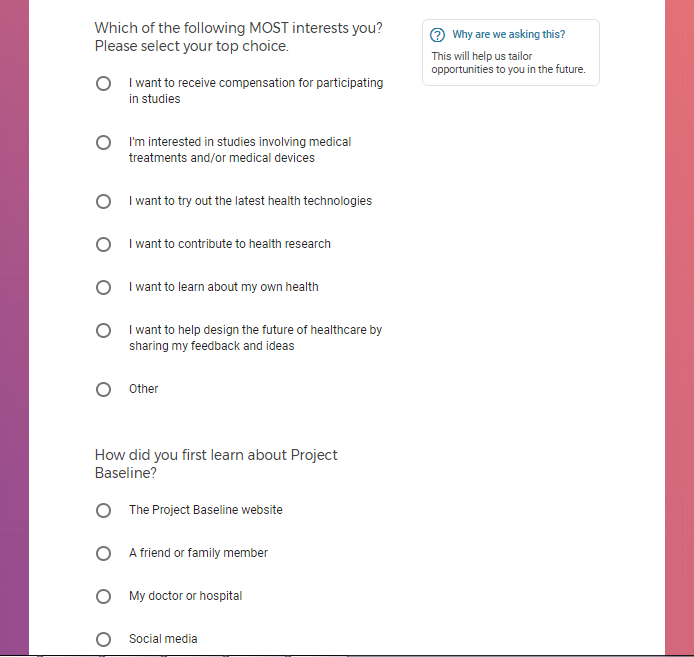
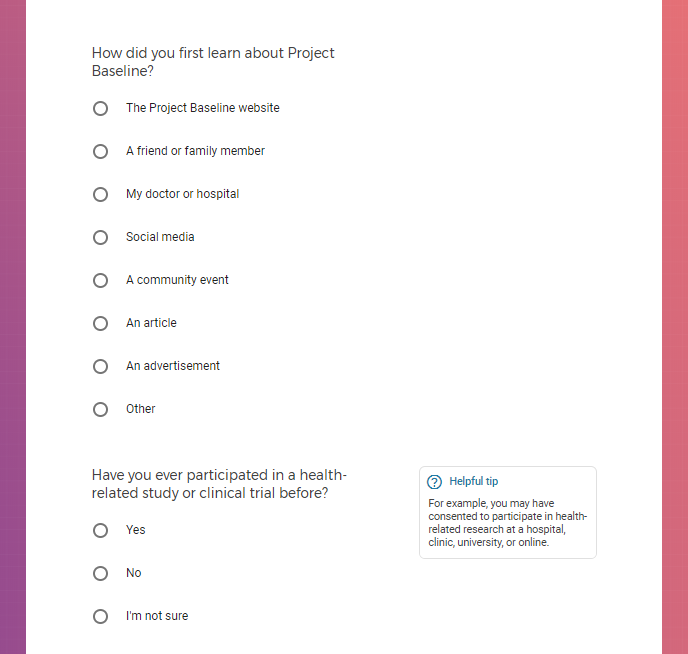
Each study you apply for adds data to your profile, which determines which studies are presented to you.
Not seeing a COVID-19 study, I applied for every study I was presented with.
( aka "Clinical Depression")
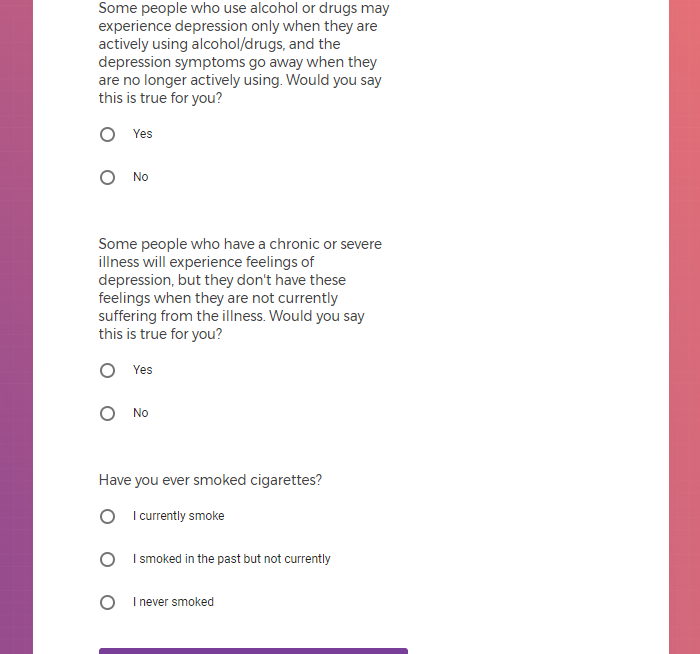


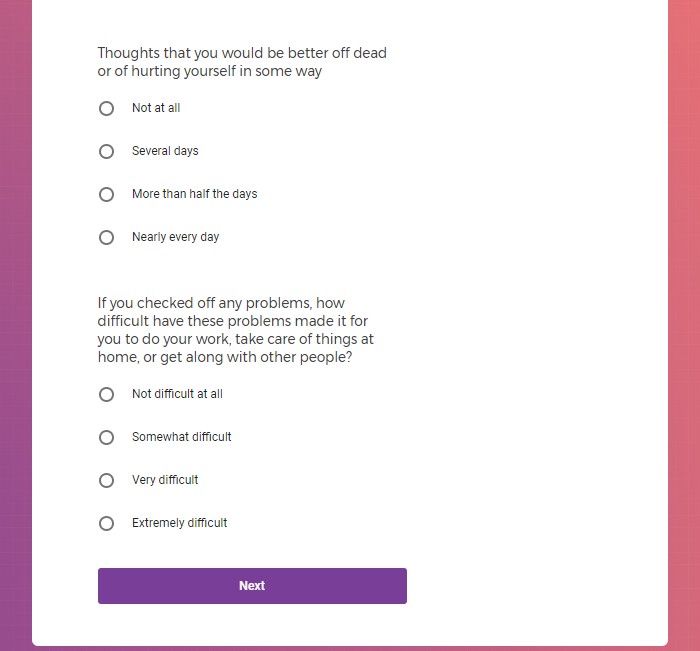
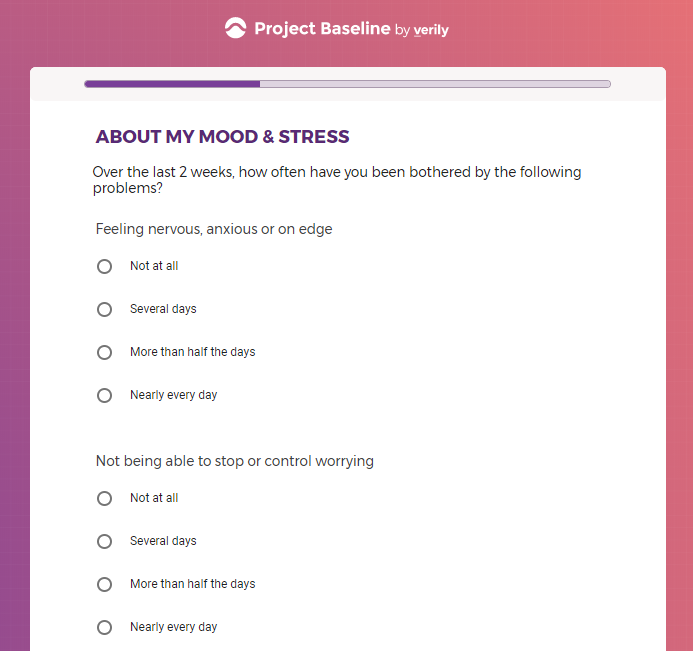
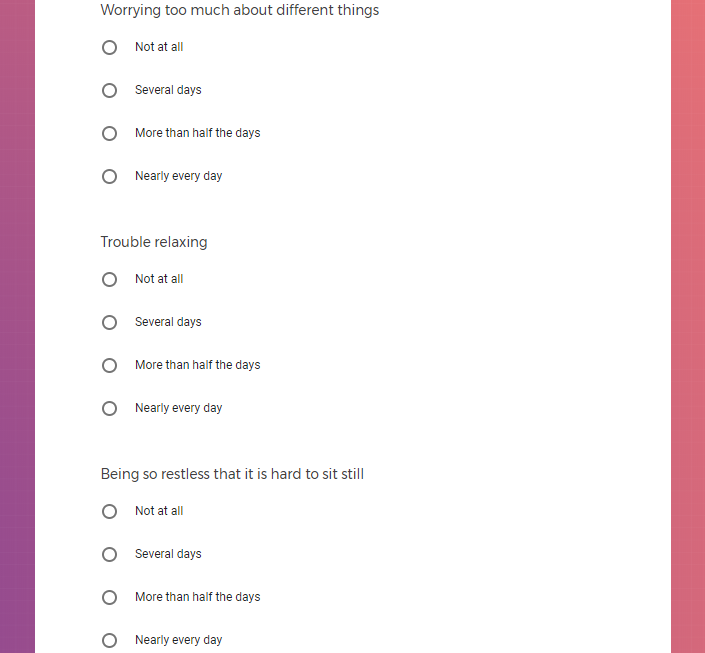

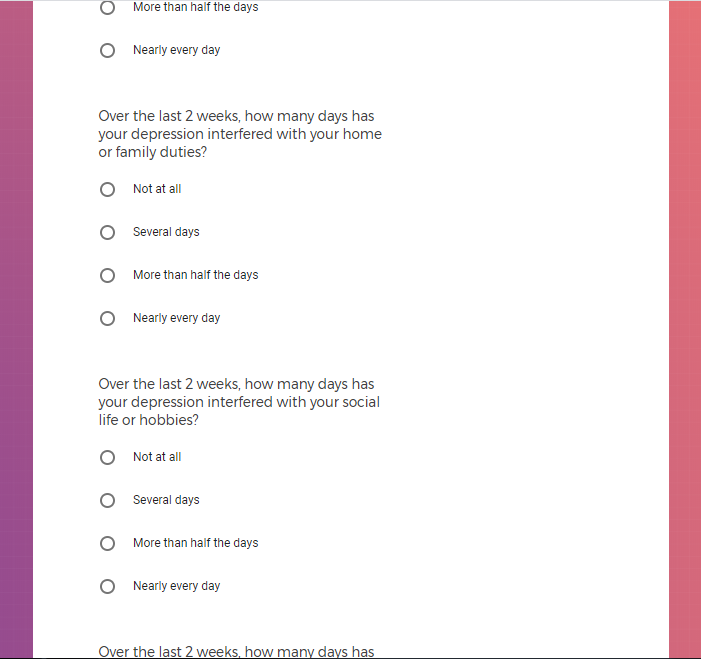
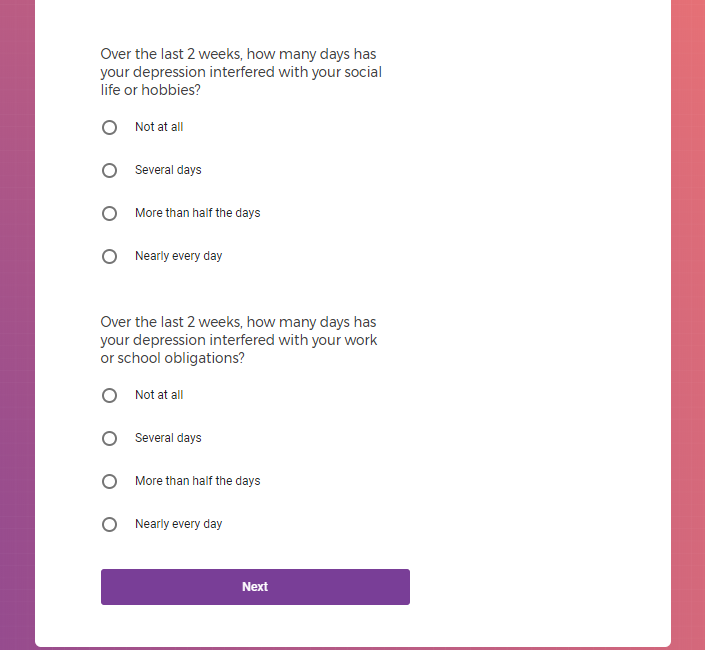
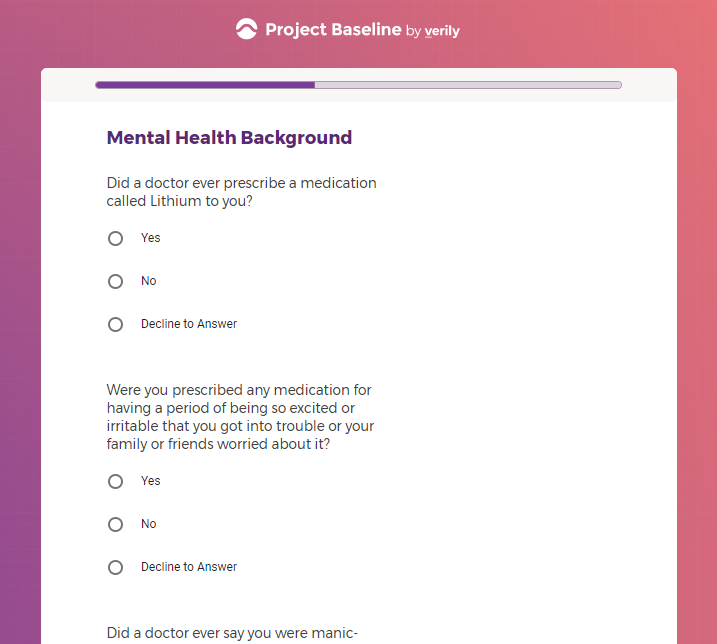
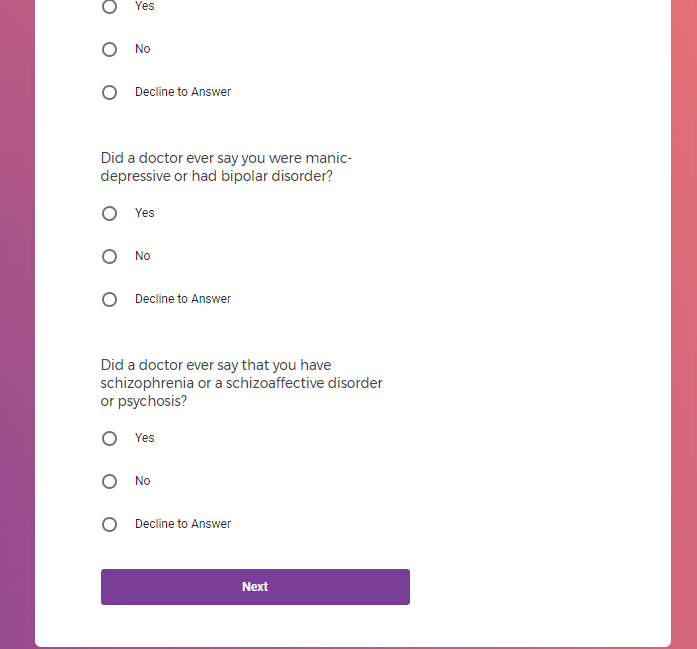
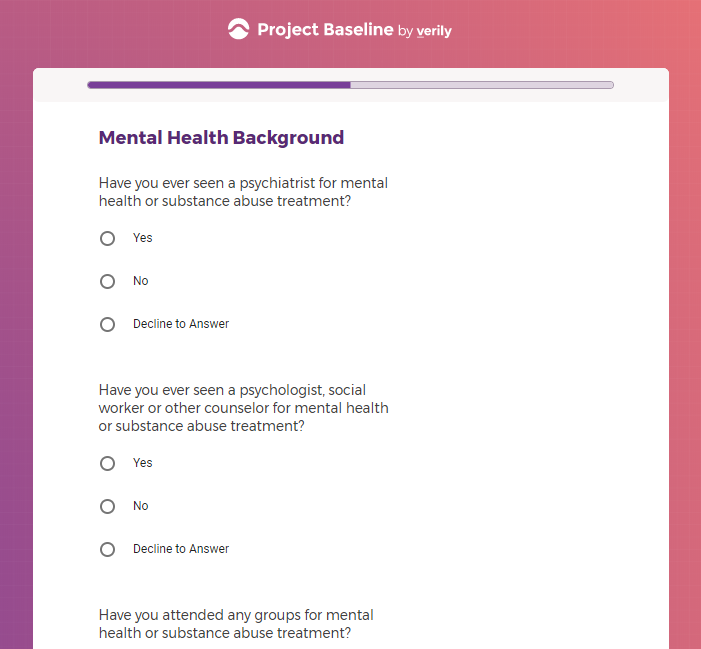
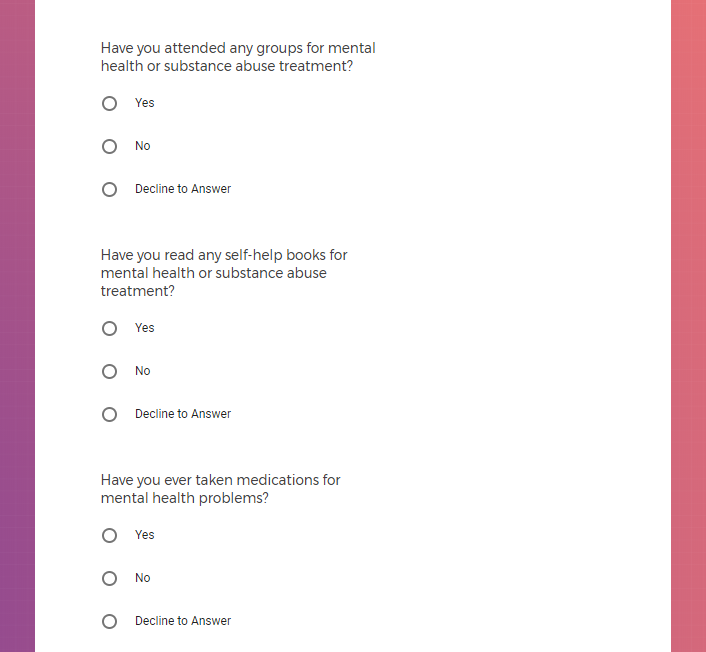
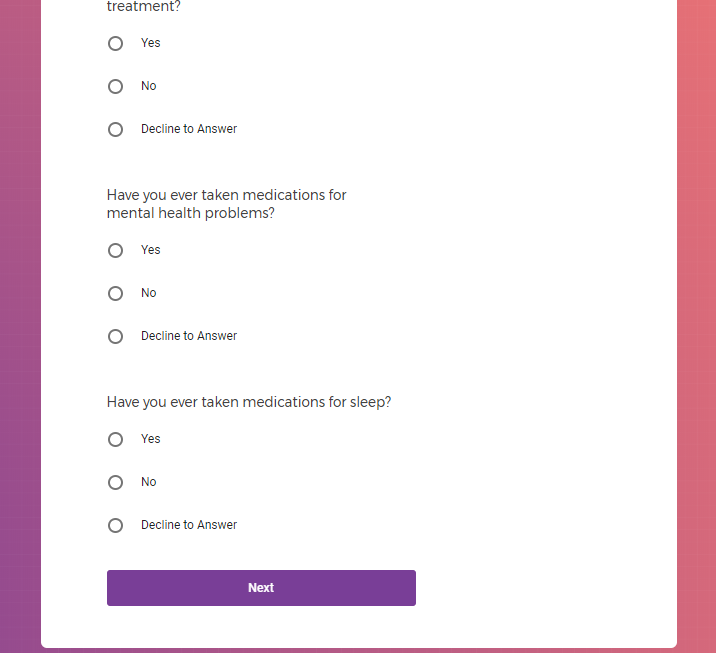
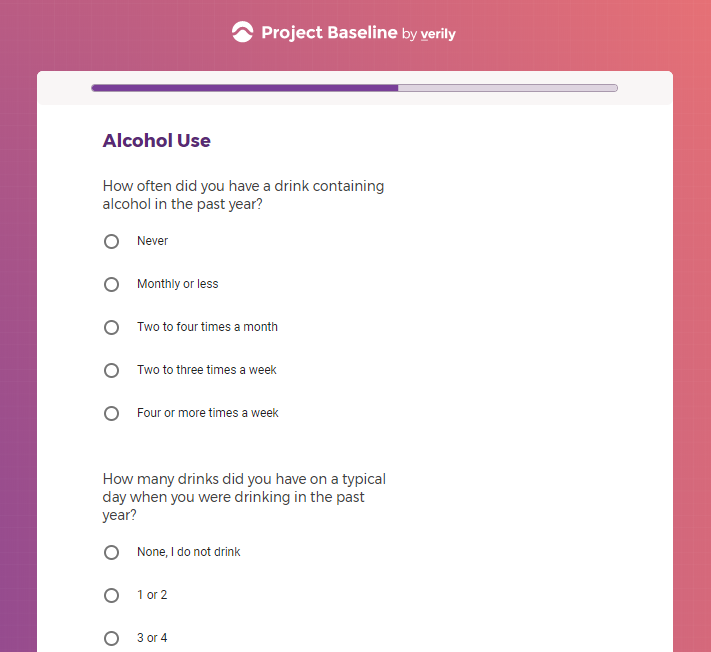
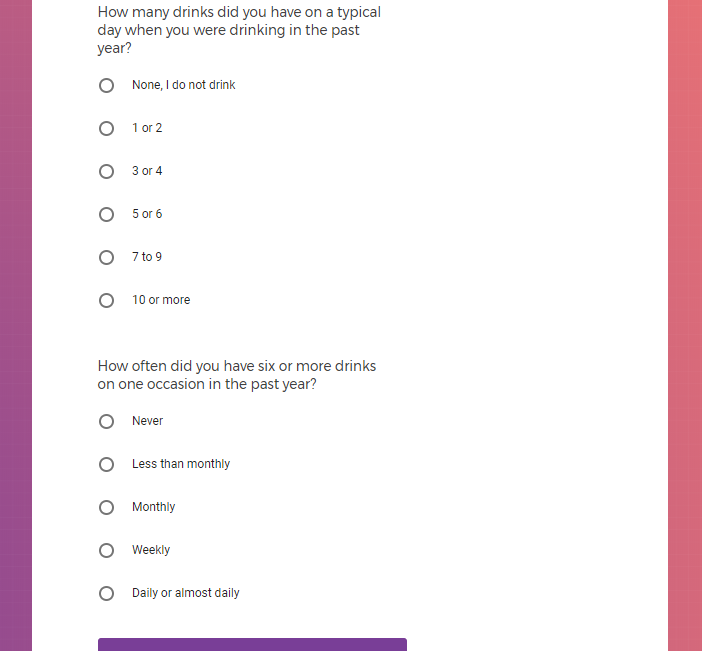
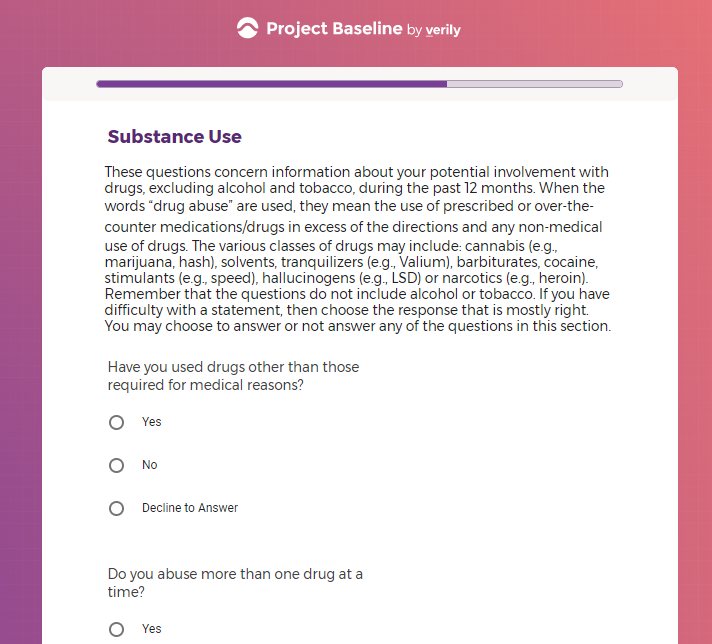
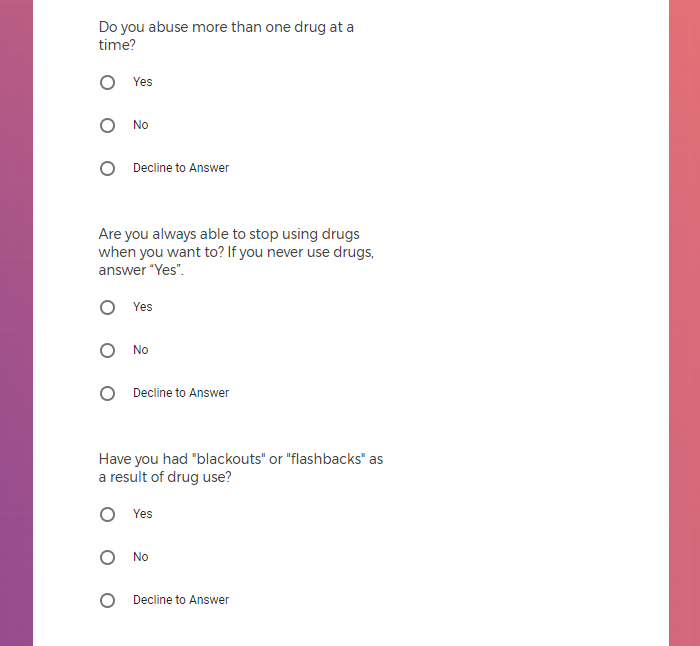
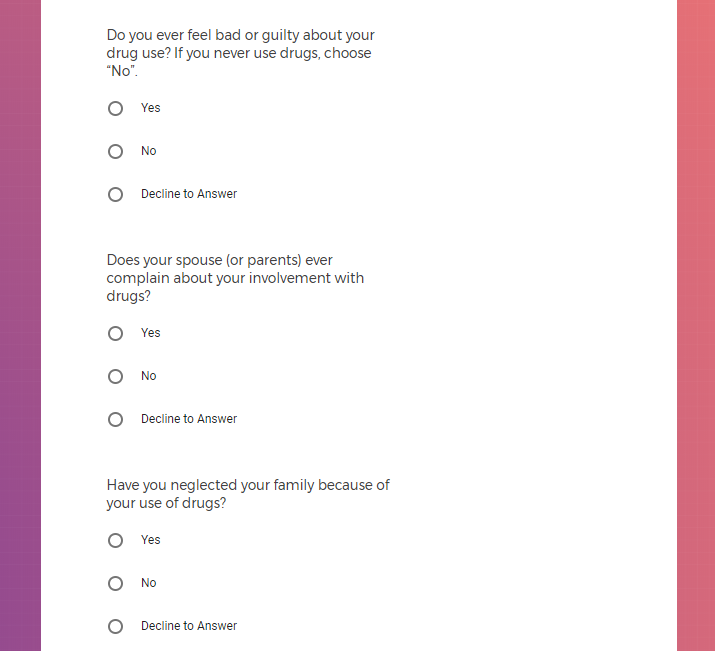
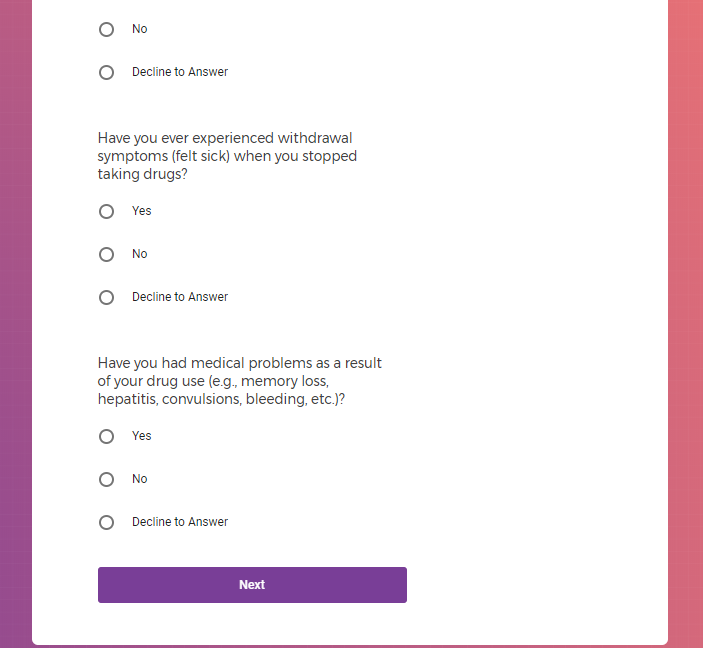
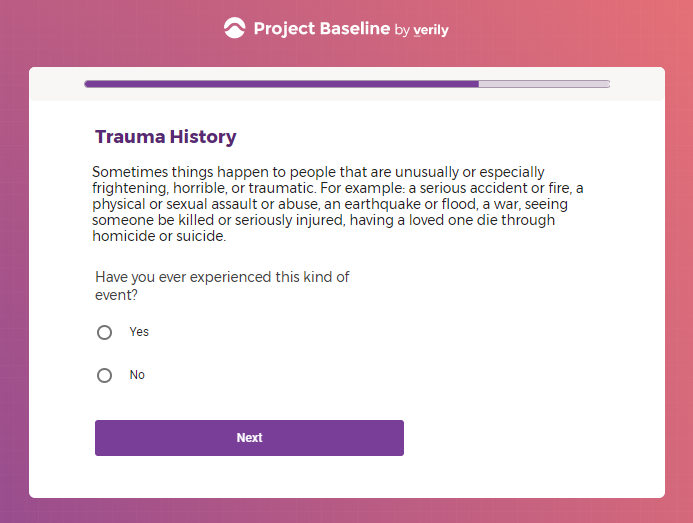
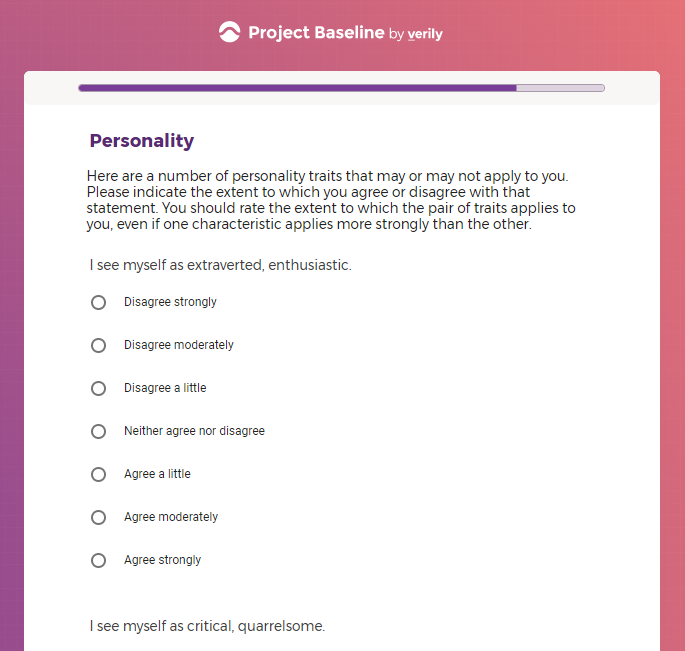
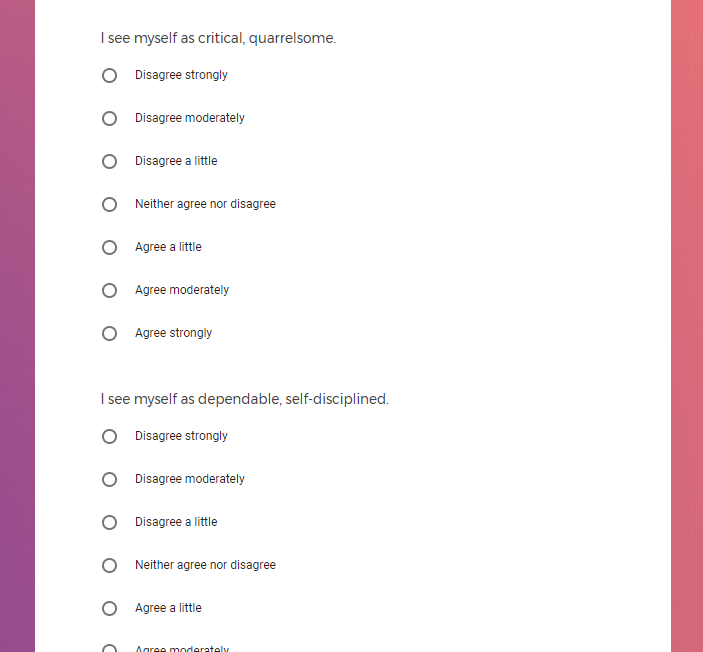
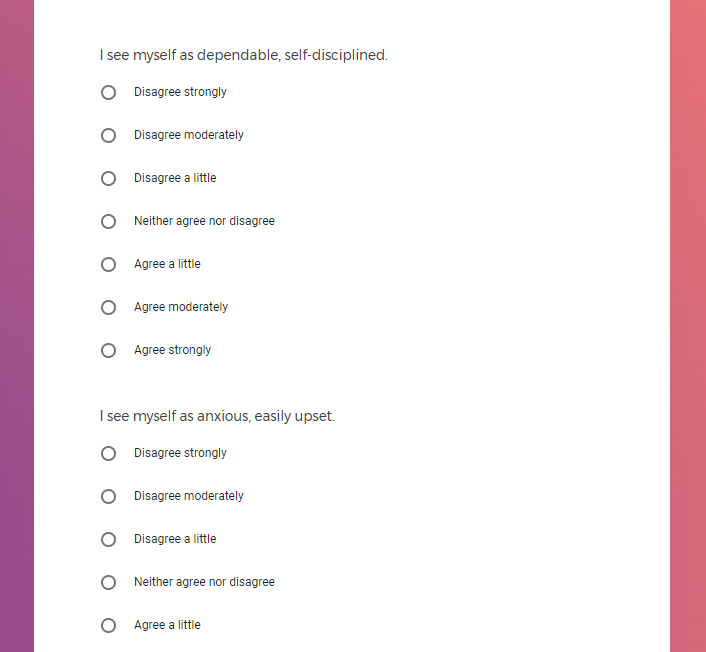
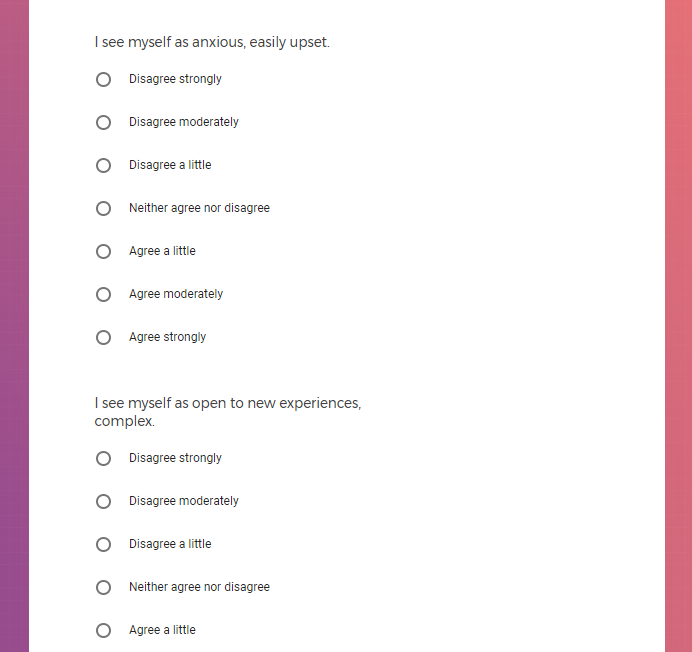
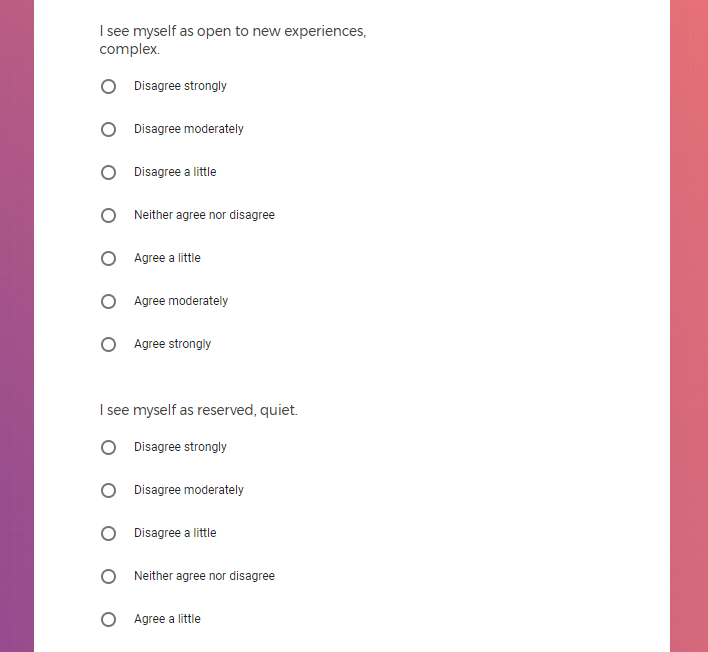
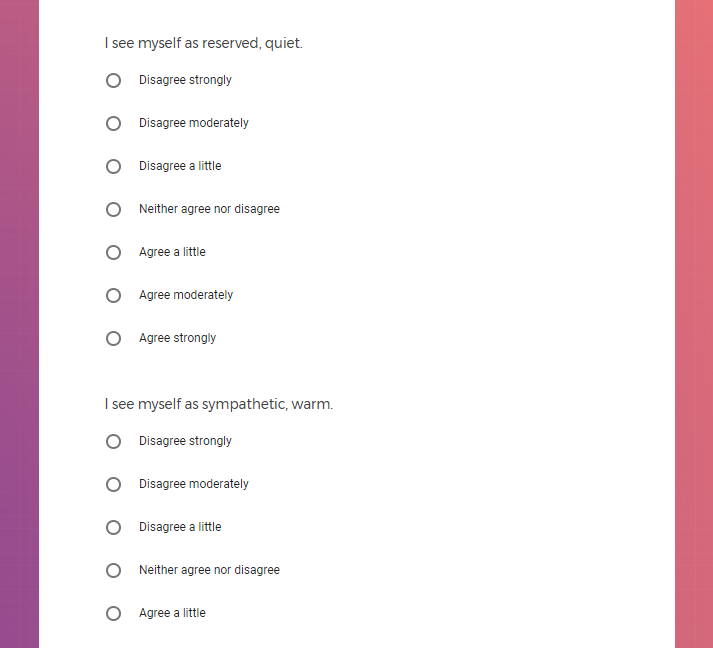
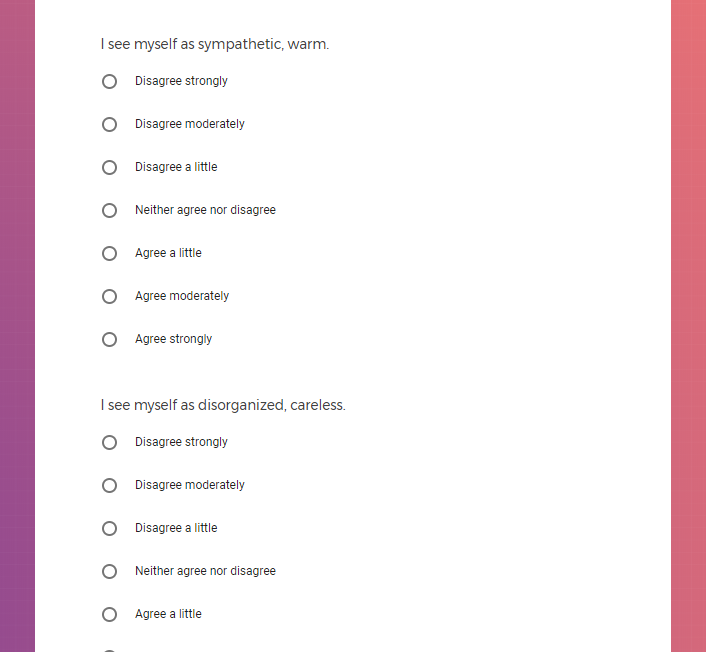


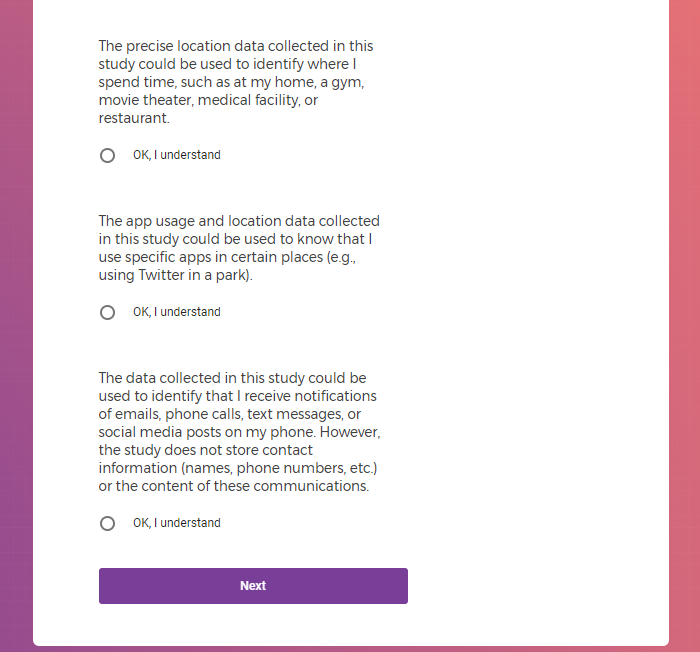
In addition to Verily's Privacy Policy, Verily's Terms of Service, Baseline Privacy Policy, and Baseline Terms of Service,
additional consents were collected in the study application intake questionnaires in the form of questions,
and additional notice provided in tooltips for some questions,
there is also an additional Consent for some studies, such as Mood Study #2.
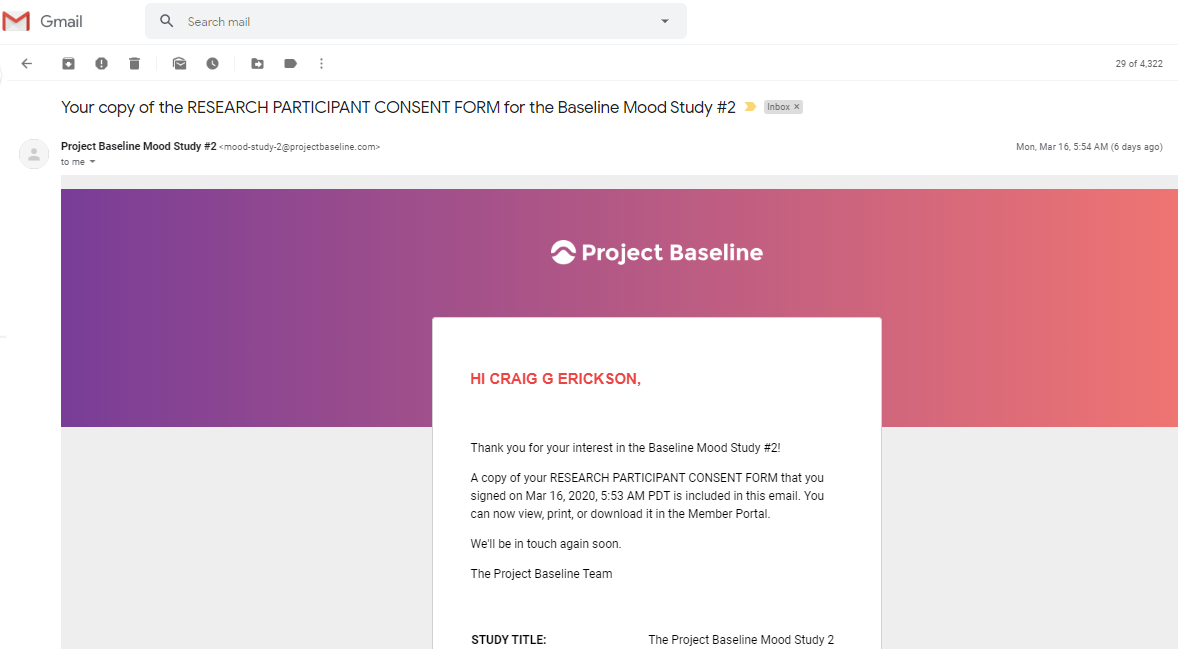
This is the body of the content included in the email:
STUDY TITLE: The Project Baseline Mood Study 2
STUDY PROTOCOL NO.: 101782
WIRB® Protocol #20192893
SPONSOR: Verily Life Sciences LLC
INVESTIGATOR: Justin Baker, MD, PhD
269 East Grand Avenue
South San Francisco, California 94080
United States
STUDY-RELATED PHONE NUMBER(S): The Mood Study 2 User Support Center
833-391-8633
Email: mood-study-2@projectbaseline.com
SUMMARY
A person who takes part in a research study is called a participant. In this consent form, "you" always refers to the participant. You are being asked to decide if you want to join the Project Baseline Mood Study 2, which is a part of the Project Baseline Community Study that you have previously joined. This consent form may add to or change the information in the consent form you signed at the start of this study. The purpose of this consent form is to help you decide if you want to participate in the research study. It explains what will happen during the study, why it is being done, and your rights as a research participant.
You have the right to know what will happen during this study, as well as the possible risks and benefits. Signing this form will mean that you agree to participate in the research study. Your participation in this study is entirely voluntary and you may decide not to participate or you may leave the study at any time. If you do not wish to participate or if you decide to leave the study at any time, this will not affect any current or future care or associated benefits or treatment that you receive from your doctor.
Things to know before deciding to take part in this research study:
The main goal of this research study is to learn things to help patients in the future.
Participation in this study will not provide you with any form of healthcare or health advice. This study is not a substitute for your usual medical care. If you choose to take part in this study, you should continue with your usual medical care and contact your health care provider(s) for any health-related questions or concerns.
This consent form may contain scientific terms or other words that are unfamiliar to you. Please contact the Mood Study 2 User Support Center at the number listed above to ask the study staff to explain anything that you do not clearly understand. You should take your time to decide whether or not you want to join this research study. You should not join this research study until all of your questions are answered.
Please read this form carefully. Additional information about this study can be found on the Mood Study 2 website: www.projectbaseline.com/study/mood2.
This study will enroll up to 32,000 people nationwide who are suffering from depression. The duration of the study is 12 months.
By signing this form, you agree to take part in a study where you will be provided with a mobile software application for your phone, called the Mood Study App. By signing this form, you agree to follow all instructions provided to you by Verily, the sponsor of the study, and the study staff. You will not replicate or copy the Mood Study App or allow anyone else to do so. You will not sell, loan, or give away the Mood Study App to anyone else. Verily retains title, ownership, and all rights to the Mood Study App and anything else that you are provided in connection with the study. By signing this form, you agree that (a) you will not use the Mood Study App outside of what is described in the "Procedures" section below; and (b) you will uninstall the Mood Study App on the date specified, or immediately upon request, or when you no longer participate in the study.
PURPOSE OF THE STUDY
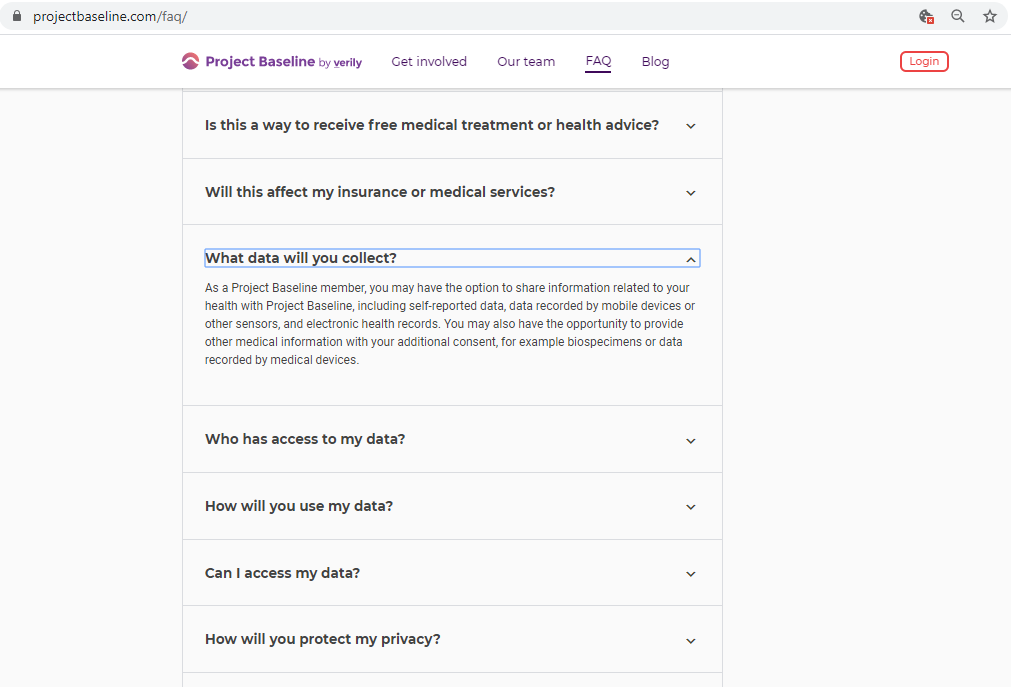
The purpose of this study is to determine better ways to collect information about an individual's mental health, using a smartphone application called the Mood Study App. The Mood Study App collects and stores information related to a user's mental health, including mood, physical activity, social function, and sleep patterns. The Mood Study App automatically collects data through sensors in your smartphone (for example, a sensor for location or ambient light), as well as active data entry from the user, such as surveys and diaries.
The information collected in this study will not be used to diagnose medical conditions or make treatment decisions.
STUDY ELIGIBILITY
In order to participate in this study, you will need to meet certain eligibility criteria.
You are eligible if you:
Are an adult (i.e.,18 years of age or older, unless a resident of any U.S. state where the age of majority is 19, in which case you must be 19 years of age or older);
Are a legal U.S. resident with a Social Security Number (used for ID verification);
Are able to read and speak English;
Are able and willing to provide informed consent for this study and the Baseline Community Study;
Have a Google user account or be willing to create an account;
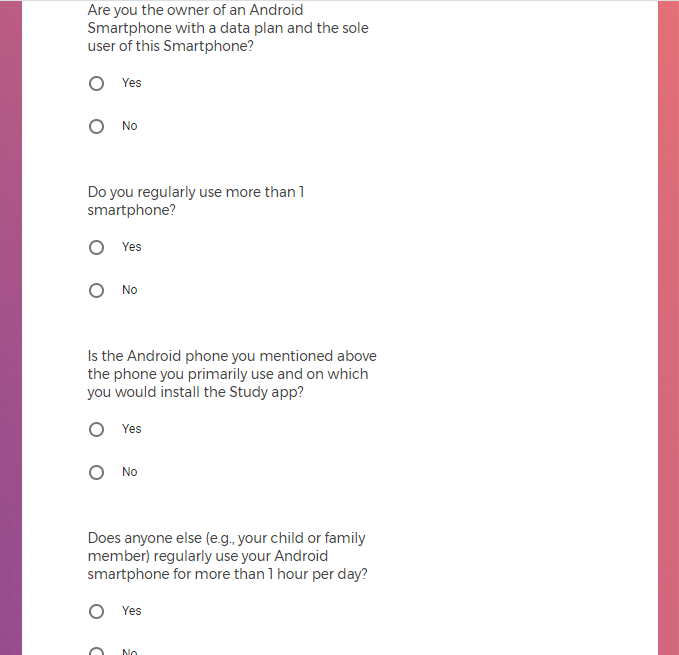
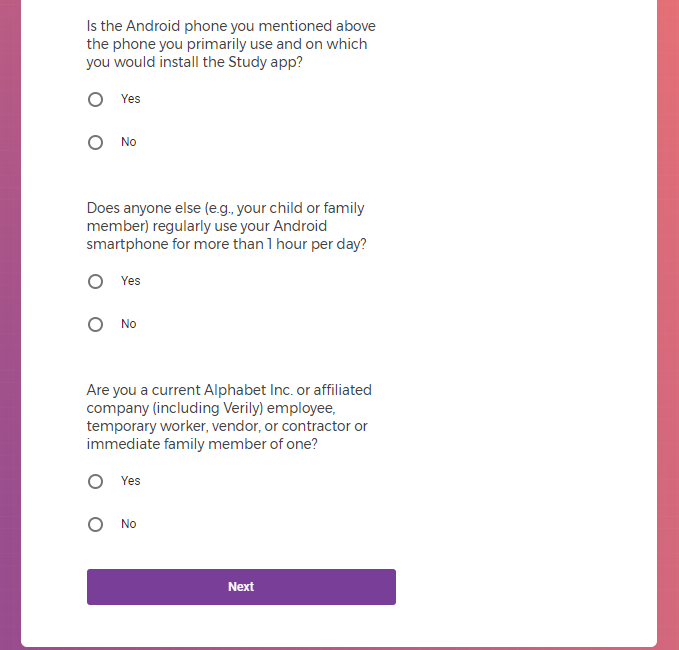
Own an Android smartphone with a data plan and are the sole user of that smartphone;
Agree to download the Mood Study App onto an Android phone;
Agree to provide a phone number and email address to enable feedback through communication.
Are currently depressed and/or in treatment for depression (with medication and/or therapy)
Started a medication and/or therapy for depression within the past 2 weeks, stably in treatment for at least 12 weeks, or not currently in treatment
Are able and willing to comply with all study procedures
You are not eligible if you:
Are a current employee, temporary worker, vendor, or contractor of Alphabet Inc. or affiliated company (including Verily) or immediate family member of one.
Report active suicidal thoughts and also report having a plan to harm yourself.
Have any other condition or situation the Principal Investigator determines as inappropriate for study inclusion.
This study involves no in-person visits. All study procedures will be conducted remotely through the Project Baseline web site and your personal Android smartphone only. If you agree to take part in this study, you will be asked to sign this consent form. A copy of the signed form will be sent to you by email.
PROCEDURES
Screening Survey
After you sign this consent form, you will be asked to use the Project Baseline Mood Study 2 Web Portal to complete a screening survey which asks about things such as:
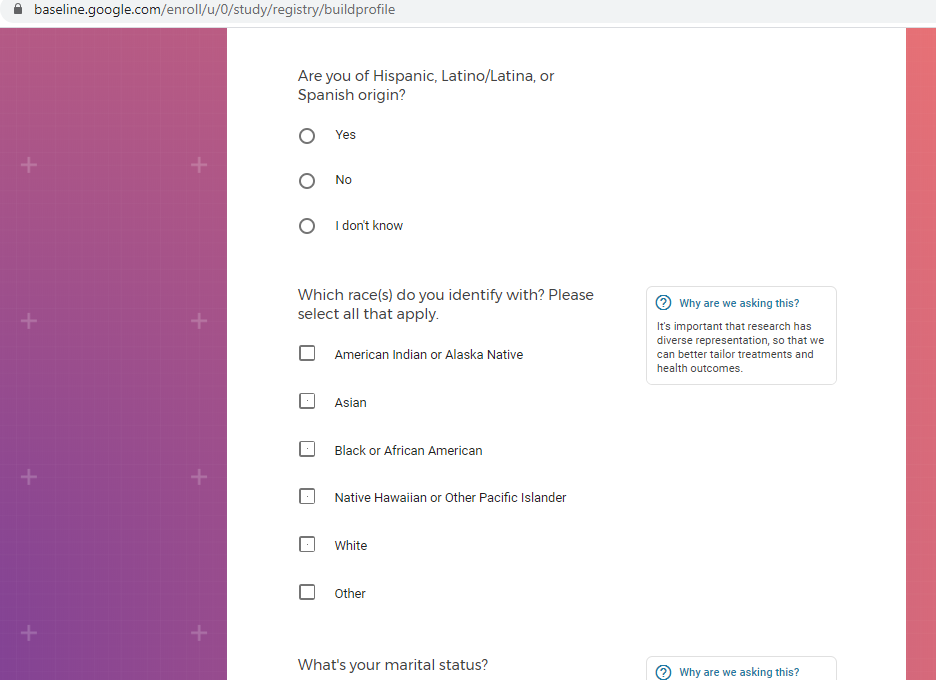
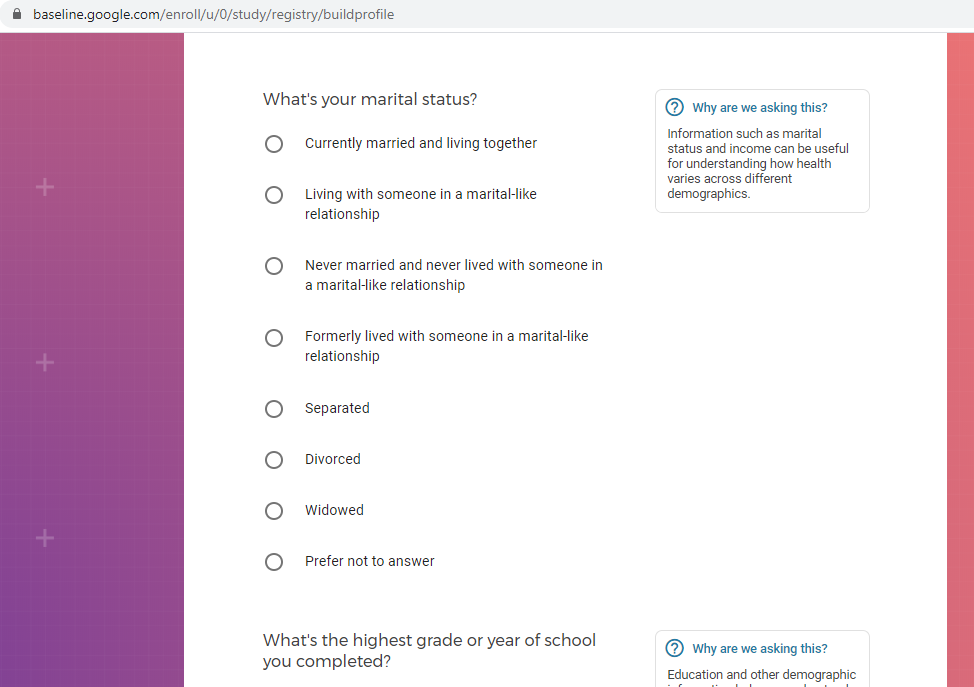
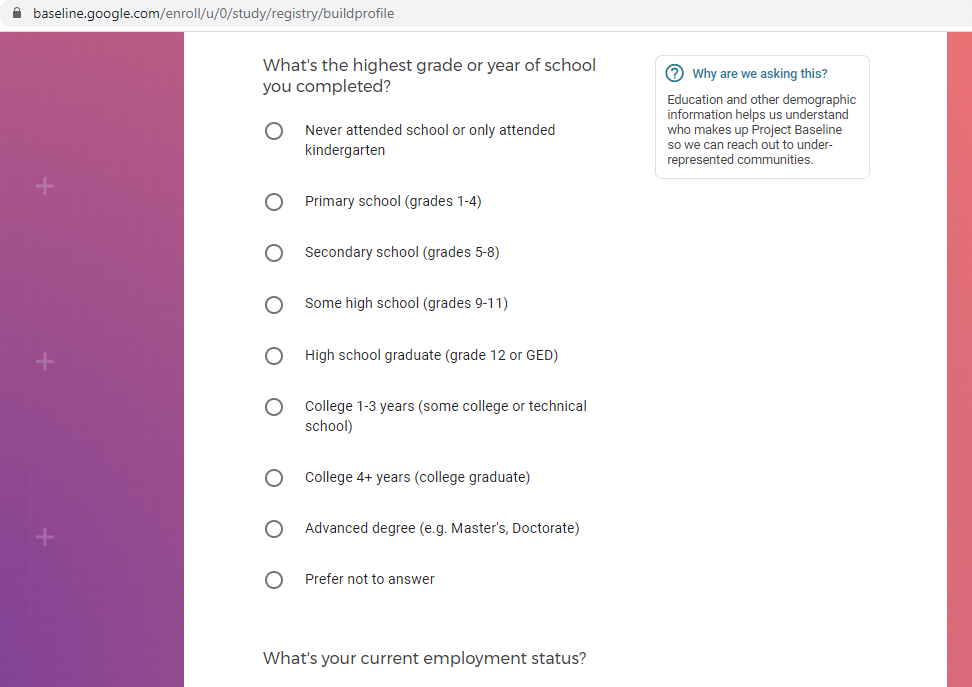
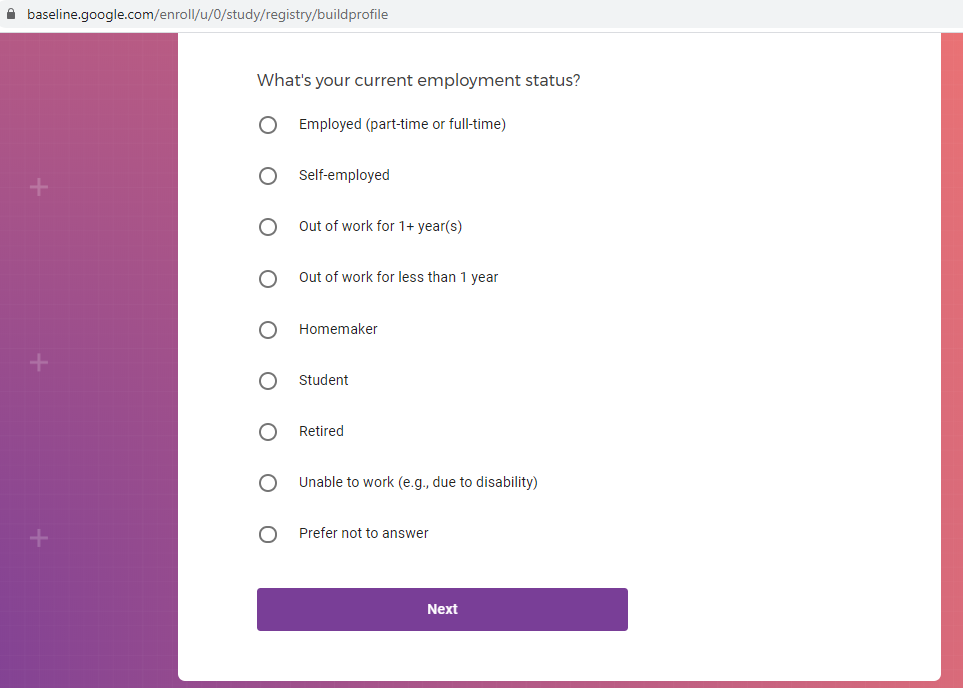
Demographic information, such as your race, sex, gender, occupation, marital status, education, and salary range.
Information about the type of mobile phone you own.
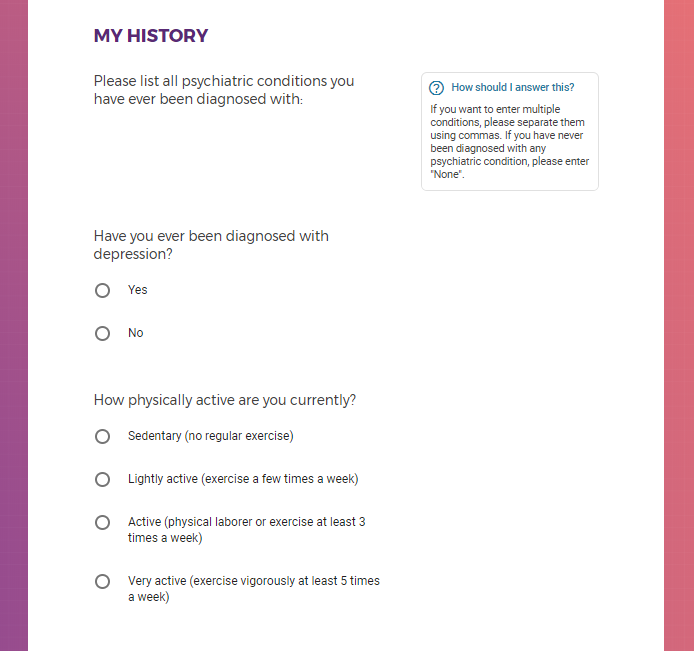
Mental health information, including previous or current treatment for depression, including medications received.
Additional mental health history, such as anxiety, drug use, alcohol use, and post traumatic stress disorder (PTSD).
This screening survey will be used to:
Determine if you are eligible for the Mood Study 2.
Understand characteristics of eligible and non-eligible individuals.
Notify you of future studies that you might be interested in.
Enrollment into the Study
Approximately a few hours after you complete the screening survey, we will notify you by email whether or not you are found to be eligible for the study. If you are eligible, the email will instruct you on how to download the Mood Study App onto your Android smartphone.
If you need help with downloading or using the Mood Study App, you will be able to request that a Mood Study 2 User Support Center team member contact you through your email address or phone number.
After you download the Mood Study App, you will be asked to use the app to complete all future surveys and study tasks described below. You may receive reminders via the app, email, phone call, and/or text message to complete tasks.
The data collected in this study, as outlined below, may be used alone or in combination with other health information collected as part of the Project Baseline Community Study.
Study Tasks: Intensive Assessment Period (Initial 12 weeks)
During the first 12 weeks of the study, known as the Intensive Assessment Period, you will be asked to complete various activities through the Mood Study App. Below is an outline of the name, description, and frequency of the activities; note that if you do not respond to the survey when prompted, you may be re-prompted with the same survey on subsequent days. A "Sprint" refers to a one or two week focused time period during which we will ask detailed questions about behaviors relevant to understanding depression.
Name of Activity Brief Description Frequency
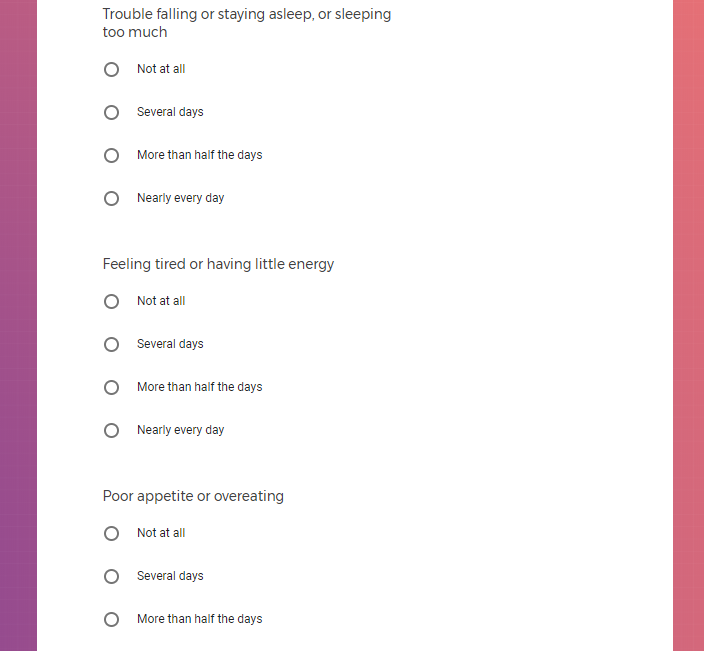
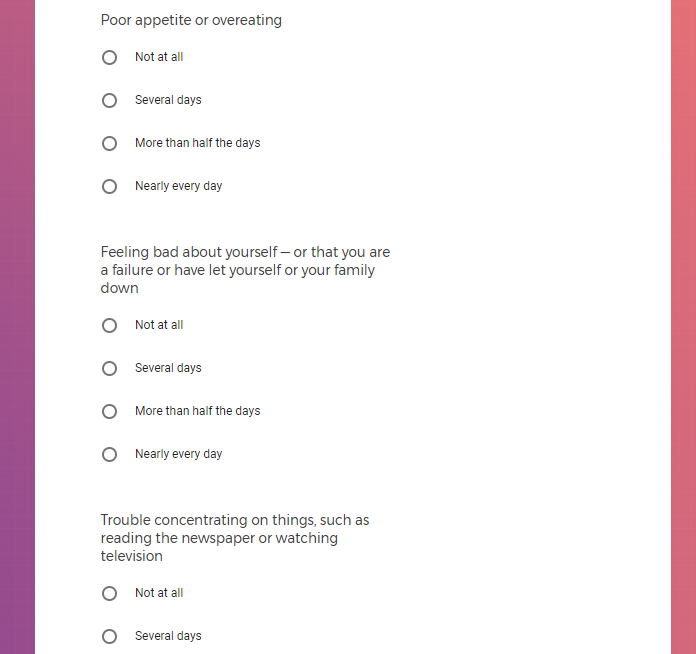
Mood Questions Three brief questions about how energetic, stressed, or depressed you felt the previous day. Daily
PHQ-9 and GAD-2 Survey You will be asked to answer clinically validated survey questions to evaluate depression and anxiety severity using the PHQ-9 and Generalized Anxiety Disorder (GAD-2) surveys. Weekly, for initial 12 weeks, and then every 15 days for the next 9 months
Quality of Life (QoL) Survey You will be asked about how depression impacts your daily life, about recent visits to a healthcare professional, and changes to your depression treatment. Weekly, for initial 12 weeks, and then every 15 days for the next 9 months
Voice Diary You will be prompted to record a voice diary describing your week so far. In addition, you will be prompted to read a specific passage of text. These voice diaries may be manually reviewed for the purpose of quality control or improving algorithm development. Weekly, for 12 weeks
Text Diary You will be prompted to share any notable events in the last week via text input. In addition, you will be prompted to type a specific passage of text. These text diaries may be manually reviewed for the purpose of quality control or improving algorithm development. Weekly, for 12 weeks
Physical Activity Sprint You will be prompted to answer questions about your physical activity over the last day. Daily, for 1 week only
Location Sprint You will be prompted to answer questions about your locations over the last day. Daily, for 2 weeks only
Sleep Sprint You will be prompted to answer questions about your sleep quality over the past day and night. Daily, for 2 weeks only
Social Sprint You will be prompted to answer questions about your social activity over the last day. Daily, for 1 week only
Treatment You will be prompted to answer questions about your current treatment for any medical conditions. Up to 3 times during initial 12 weeks
Medication Photo You will be asked if you are taking any medication for treatment. If yes, you will be asked to upload a picture of the medication bottle. This photo may be manually reviewed for the purpose of quality control or improving algorithm development. Up to 3 times during initial 12 weeks
General Questions You will be asked some general questions, for example about your work schedule or how you interact with your phone Up to 3 times during initial 12 weeks
Study Tasks: Low Intensity Assessment Period (12 weeks through 12 months)
After the initial 12-week intensive assessment period, the study will transition into a lower intensity assessment period, consisting of only occasional surveys while the Mood Study App continues to collect sensor data passively in the background.
Every 15 days, you will be asked to complete the PHQ-9 and GAD-2 surveys, as well as the Quality of Life (QoL) Survey; see above for more details on these surveys. Over the course of these 9 months, you will be asked to complete these surveys a total of 18 times; note that if you do not respond to the survey when prompted, you may be re-prompted with the same survey on subsequent days.
Study Tasks: Participant Feedback
We will send you an email asking for your feedback on the Mood Study App and study participation after completing the study tasks at months 1, 3, 6 and 12; note that if you do not respond to the survey when prompted, you may be re-prompted with the same survey on subsequent days.
Passive Data Collection
The Mood Study App will automatically collect information from your phone and sensors on your phone as long as the app is on your phone throughout your participation in the study. If you agree to participate in the study, the Mood Study App may collect some types of information stored on your phone starting up to 1 week prior to your enrollment in the study up to the time the Mood Study App is uninstalled from your phone. While the app is installed, the collection of information will be ongoing, unless you log out of the app or remove the app from your phone. If you log back into the app after logging out of the app or uninstalling it, the app may collect some types of information stored on your phone from up to 1 week prior to your logging in.
Some information collected may be analyzed to help create a better understanding of how data collected from your smartphone relates to your mental health. For example, information related to your location may be compared to additional location data from Google Maps about the types of businesses or places located nearby. The collected information will not be analyzed in real-time and will be performed after the study ends to see if they can help predict mood changes or other outcomes of interest. These analyses will not be used to identify you personally, although they may inadvertently reveal information that could identify you, for example the types of places you have visited. Before giving analysts access to your data, name and contact information will be removed. Additional information regarding the privacy and security of your data can be found in the "CONFIDENTIALITY" section.
The type of information collected and the time of collection are detailed in the table below. Please review the table carefully.
You may be contacted by the Mood Study 2 User Support Center if the Mood Study App on your phone is not collecting passive data properly.
Information Collected ("Sensor") Privacy Considerations Collection Start Time
Light Level: Ambient light level detected by the phone There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Pressure: Ambient air pressure measured by the phone There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Proximity: Measurement of whether an object is within a given distance. This can be used to help determine if the phone is in a pocket or bag. There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Step Count: Estimated number of steps taken. This data comes from the phone itself or wearable devices connected to your phone through Google Fit. There is minimal risk that this information can be used to identify you. Up to 1 day prior to the first time you log into the Mood Study App
Accelerometer: Measures the tilting motion and orientation of your phone There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Gyroscope: Adds a dimension to the information supplied by the accelerometer by tracking rotation or twist There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Magnetometer: Used to approximate the direction your phone is pointed in (e.g., North) There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Activity Recognition: Estimated activity from a predefined set of activities (e.g., walking, running, biking, sitting). There is minimal risk that this information can be used to identify you. Up to 1 day prior to the first time you log into the Mood Study App
Location: Estimated latitude and longitude (GPS coordinates) of your phone It may be possible to identify your recent locations. After you log into the Mood Study App for the first time
Audio Output: Volume settings on your phone for different sounds (e.g., alarm, music, ringer). There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Bluetooth: Bluetooth devices in the vicinity and if bluetooth is enabled on your phone Bluetooth device names are assigned identification numbers so the actual bluetooth device names are hidden. After you log into the Mood Study App for the first time
Device: Information on your phone and operating system There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Notifications: Monitors social contact and network information via Android notifications for other apps (e.g., emails, phone calls, text messages). Data from this sensor provides the count and timestamp of deidentified notifications. Identifying data (e.g., email addresses, phone numbers, and names) of people with whom you have contact are assigned identification numbers so that the actual data is hidden. After you log into the Mood Study App for the first time
Network / Traffic / Usage Stats: Tracks which apps are running on your phone and for how long. Also measures network bandwidth usage by app. Only the names of Google apps and apps from Play Store are tracked. Specific names of apps are captured, but what you do within these apps is not captured. Up to 1 week prior to the first time you log into the Mood Study App
WiFi Networks: Names of WiFi networks in the area (not just the one connected to your phone), and if WiFi is enabled on your phone WiFi network names are assigned identification numbers so that the actual WiFi network names are hidden. After you log into the Mood Study App for the first time
Network Sensor: Tracks the network your phone is connected to and the network type (data plan versus WiFi network) Network names are assigned identification numbers so that the actual names are hidden. After you log into the Mood Study App for the first time
Phone Calls: Logs summary data about incoming / outgoing phone calls Phone calls are never recorded. Only summary data on phone calls is collected (start time, stop time, missed call) After you log into the Mood Study App for the first time
Battery Charge: Measures when the phone is charging and how full the battery is There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Screen State: Measures when your phone's screen is on or off There is minimal risk that this information can be used to identify you. After you log into the Mood Study App for the first time
Permissions: Measures when you have declined to allow permissions for the app to gather data from certain sensors There is minimal risk that this information can be used to identify you. Measured once a day, after you log into the Mood Study App for the first time
Sleep API: Measures your environment's ambient light, ambient audio, accelerometer data, and alarm timing to estimate sleep timing There is minimal risk that this information can be used to identify you. If other sensor types are added to the Sleep API, this data stream will be disabled. Measured every 6 minutes, after you log into the Mood Study App for the first time
Amplitude of Ambient Noise: Measures soundwaves from your microphone There is minimal risk; sensor captures only average ambient sound amplitude readings. Speech or audio content is not stored. These sensors are disabled during all phone calls or when any other app is using the microphone. Measured every 60 seconds for 600 milliseconds, after you log into the Mood Study App for the first time.
STUDY DURATION
If you agree to participate in this study, your participation will last up to 12 months. The 12 months of participation are made up of two parts:
Intensive Assessment Period: 12 weeks of engagement consisting of active surveys (as detailed above), and passive data collection through the Mood Study App.
Low Intensity Assessment Period: 9 months of engagement consisting of active surveys every 15 days, and passive data collection through the Mood Study App.
RISKS AND DISCOMFORTS
By using the Mood Study App, you may be exposed to the following additional risks:
You may feel uncomfortable answering some of the assessment questions or you may feel fatigued by an assessment. You may decline to answer any question or discontinue the assessment at any time.
If someone other than yourself is able to access your phone (for example, if your phone is not locked via password protection and you leave it out of your care), your responses to some assessments may be seen. You should not use someone else's device to participate in this study.
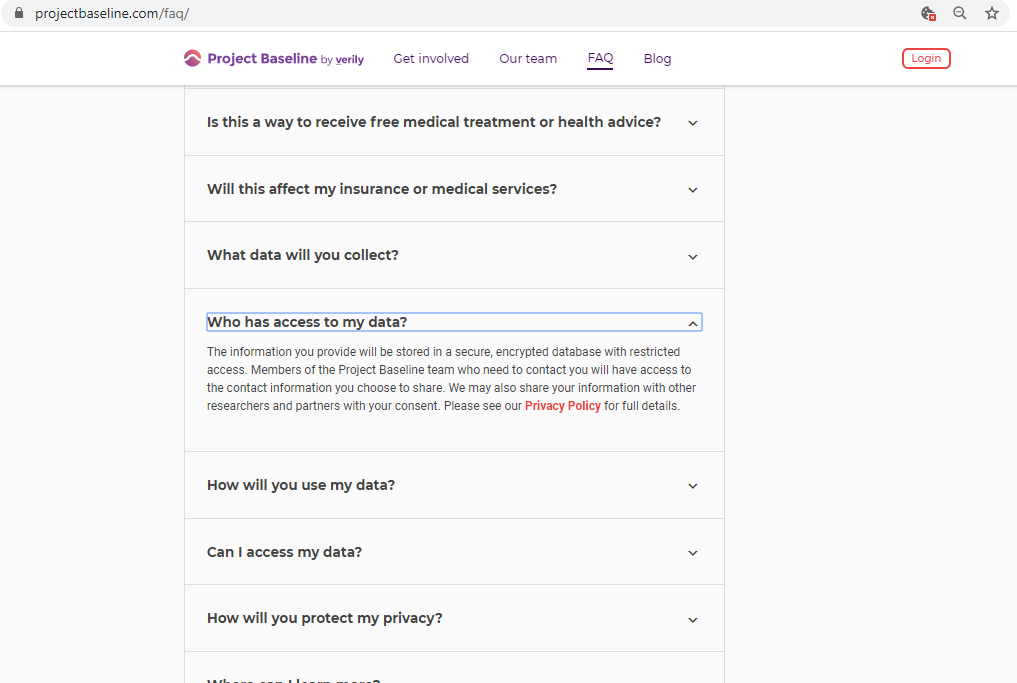
When you agree to create a Study Profile and use the Mood Study App, you provide Verily Life Sciences and the Mood Study Team with access to information about you. Verily will keep health information on secure systems with many layers of protection. However, there is a risk that someone could get unauthorized access to the information stored about you. Taking part in this study requires the use of one or more of an external study website, mobile applications, messaging, and email. Because some of these systems are developed and managed externally, there is no guarantee that they are free of risk. Additional information regarding the privacy and security of your data can be found in the "CONFIDENTIALITY" section.
NEW INFORMATION
You will be told about any new information that becomes known to researchers that might change your decision to be in this study. You may be asked to sign a new consent form if this occurs.
BENEFITS
There is no direct benefit to you for participating in this study; however, the information gained from this study may help others in the future.
You will not have any rights to licensing or receive financial compensation from any products that may result from participating in this study.
COSTS
The study procedures (Mood Study App) will be provided to you at no cost. You will not incur any additional costs by agreeing to participate in this study. Depending on your mobile phone plan, you may incur data charges for accessing the Mood Study 2 Web Portal and Mood Study App.
RESEARCH RESULTS
You will not have access to any information collected in this study nor will you receive feedback about your survey results. You may receive an automated message to seek assistance if your responses indicate a risk of suicide or severe level of depression (though this cannot be guaranteed).
PAYMENT FOR PARTICIPATION
You will be paid for every two weeks that you complete certain tasks in this study. Your compensation will vary from week to week, depending on what tasks you are asked to complete each week. If you complete all tasks in the study, you will be paid a total of approximately $180. If you do not complete the entire study, you will be paid for the tasks you have completed. In the first 12 weeks of the study, you will be compensated approximately $11 per week if you complete all tasks. In the following 9 months of the study, you will be compensated approximately $5 per month, if you complete all tasks.
Payments will be processed using a third party payment vendor named Hyperwallet. As such, Hyperwallet is independent from the study Sponsor and they will know of your participation in this study. You will set up an account directly with Hyperwallet and will be given the option to receive payment through direct bank transfer, payment card, or check. The payment card may be used anywhere Visa is accepted. Your payment card will be remotely loaded with cash based on the study procedures you have completed. Payment cards may be subject to standard fees and other terms as described in Hyperwallet's cardholder agreement. It may take up to 3 weeks to receive payment after completing the assigned tasks.
You will need to provide Hyperwallet with your name, email address, mailing address, and your date of birth. If you choose electronic bank transfer, you will need to provide your banking information. Your information will be kept secure and confidential, as described in the "CONFIDENTIALITY" section.
If the total payment for your participation in Verily Life Sciences LLC studies is greater than $600 in a calendar year, it will be reported to the Internal Revenue Service (IRS) as income and your social security number may need to be collected. In this case, you must provide your tax reporting documentation to receive payment.
ALTERNATIVE TREATMENT
This study does not provide any health treatment or benefits. If you need advice on how to treat your depression, please contact your doctor.
CONFIDENTIALITY
Data that could be used to identify you
Information about you or provided by you during the research will not be disclosed to others without your written permission, except if it is necessary to protect your rights or welfare (for example, if you are injured and need emergency care); or if it is required by law. The information about you will be kept confidential throughout the study. A unique participant identification code will be used when collecting study data.
Authorized representatives of the U.S. Food and Drug Administration (FDA) and other federal and regulatory agencies (as required), personnel from Verily Life Sciences LLC and its affiliates (which could include Alphabet companies such as Google), and the Institutional Review Board (IRB) may need to review the data collected from you for the purposes of this study and may learn your identity.
The results of this research study may be presented at scientific or medical conferences or in publications. Your identity will not be disclosed in those presentations.
Verily Life Sciences LLC may share the data collected during this study with its affiliates (e.g., Alphabet companies, such as Google) for research, product development, or other commercial purposes. Any data shared with affiliates and research partners will not be linked back to your name and contact information. During enrollment, you will have the option to authorize Verily Life Sciences to use your home address for location analyses. If you do so, we may share your home address with affiliates for location analyses.
The third-party payment vendor, Hyperwallet, will receive information about you in order to compensate you for your participation in the study. This may include your name, email address, mailing address, and your date of birth. This information will be kept secure and confidential using data encryption methods standard to banking institutions.
Confidential Information
The following shall be considered Confidential Information: (i) Verily's role in the study, (ii) any relationship that Verily may have with any other individuals or entities involved in the study, and (iii) the Mood Study App provided by Verily to you. You may not talk to the media, blog, tweet, or post publicly about the Mood Study App or your experience using the Mood Study App, or about any other Confidential Information. You must immediately tell the study staff if you are legally required to disclose Confidential Information. You must keep all such Confidential Information secret and use it only to participate in the study.
However, Confidential Information does not include information that: (i) was known to you prior to study participation; (ii) is publicly available through no fault of yours; (iii) is rightfully received by you from a third party without a duty of confidentiality; or (iv) is independently developed by you.
You must not disclose such Confidential Information to any third party and you must use a reasonable degree of care to protect Confidential Information and to prevent any unauthorized use or disclosure of Confidential Information. You may disclose such Confidential Information when compelled to do so by law if you provide reasonable prior notice to Verily, unless a court orders that Verily not be given notice.
Unless the parties (Verily or a third party) otherwise agree in writing, your duty to protect Confidential Information survives forever.
COMPENSATION FOR INJURY
The Mood Study App does not have any direct participant contact, therefore, research related injuries are not anticipated. If you believe you are injured as a direct result of using the Mood Study App, you will be responsible for seeking medical treatment. Verily Life Sciences LLC will not provide medical treatment or compensate you for any costs incurred for medical treatment.
You will not lose any of your legal rights by signing this form.
VOLUNTARY PARTICIPATION AND WITHDRAWAL
Your participation in this study is entirely voluntary. You may decide not to participate or you may leave the study at any time. Your decision will not result in any penalty or loss of benefits to which you are otherwise entitled. If you decide to participate, you are free to withdraw your consent and refuse to participate at any time. You are not required to give your reason(s) for withdrawing from the study. However, the Investigator may ask for your reason(s) to exit the study while fully respecting your rights. If you decide to no longer participate in the study, you will be asked to uninstall the app to ensure that no future data will be collected. Data collected up to the date you uninstall the app will be kept and included in the study data analysis. If you decide to no longer participate in the study, you will not receive any further payments other than for those surveys that you have already completed.
If you decide to withdraw from this study, please contact the Mood Study 2 User Support Center at the phone number or email listed on page 1 of this consent form.
Your participation in this study may also be stopped at any time by the Principal Investigator or Verily without your consent for any reason, including:
if you do not consent to continue in the study after being told of changes in the research that may affect you
if the study is cancelled
if you withdraw your consent
If you no longer meet eligibility criteria
if you not do fulfill the study requirements; or
any other reason believed to be in your best interest
SOURCE OF FUNDING FOR THE STUDY
The Sponsor, Verily Life Sciences LLC, is paying to conduct this study.
FINANCIAL DISCLOSURE
The Principal Investigator, though not an employee of Verily Life Sciences LLC, is a paid consultant by the Sponsor. The sponsor's other study staff are employees or contractors of the Sponsor. The study staff owns stock in Verily Life Sciences LLC. The Study staff's stock ownership is less than 1% of Verily Life Sciences LLC's total equity. Please ask the Investigator if you have any questions relating to this potential conflict of interest.
QUESTIONS
Contact the Mood Study User Support Center at (833) 391-8633 for any of the following reasons:
if you have any questions about your participation in this study,
if you feel you have been injured as a direct result of the study procedures, or
if you have questions, concerns or complaints about the research.
If you have questions about your rights as a research participant or if you have questions, concerns or complaints about the research, you may contact:
Name: Western Institutional Review Board® (WIRB®).
Address: 1019 39th Avenue SE Suite 120, Puyallup, Washington 98374-2115
Phone: 1-800-562-4789 or 1-360-252-2500
Email: Help@wirb.com
WIRB is a group of people who perform independent review of research. WIRB will not be able to answer some study-specific questions. However, you may contact WIRB if the research staff cannot be reached or if you wish to talk to someone other than the research staff.
Do not sign this consent form unless you have had a chance to ask questions and have gotten satisfactory answers.
If you agree to be in this study, you will receive a signed and dated copy of this consent form for your records.
If you agree to be in this study, we may contact you in the future to see if you are interested in consenting to join future studies. You are free to decline participating in those future studies.
CONSENT
My signature below means that:
I have read this consent form.
I understand that I am being asked to participate in a research study, and all my questions about the study and my part in it have been answered.
I freely consent to be in this research study, and voluntarily agree to comply with the study requirements.
I understand that I can withdraw consent at any time prior to and during the study, without any legal consequences and without any penalty to benefits to which I am entitled.
I understand that my relevant personal data may be used for the purpose of this research study.
I agree that Verily representatives, affiliates of Verily (which could include Alphabet companies such as Google), regulatory authorities, and Institutional Review Board representatives will be granted direct access to my data in this study.
I will be given a signed and dated copy of this document for my records.
By signing this consent form, I have not given up any of my legal rights.
PrivacyPortfolio conducts privacy research experiments to answer social, legal, and ethical questions such as these.
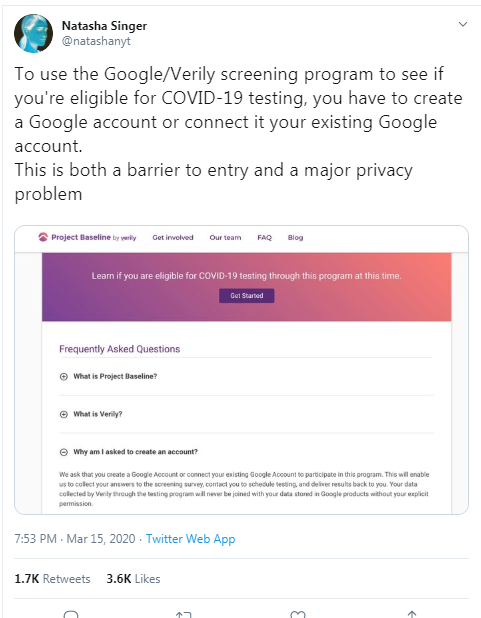

PrivacyPortfolio's research methodology publishes its tests and test results in open, public datasets for transparency and replication.
Iterating these tests over time is another way to validate the methodology used as well as the results posted as evidence.
